John Saghir1, Janet Herrada1, Lisa Long1, Erin San Valentin1, Thomas S. McCormick1, Mahmoud Ghannoum1, 2
1Center for Medical Mycology and Integrated Microbiome Core, Department of Dermatology Case Western Reserve University, Cleveland, Ohio
2University Hospital Cleveland Medical Center, Cleveland, Ohio
Mahmoud A. Ghannoum
mag3@case.edu
Saghir J, Herrada J, Long L, Valentin ES, McCormick TS, Ghannoum M. Efficacy of Rezafungin on Candida albicans Endophthalmitis in a Rabbit Model. Pathogens and Immunity. 2025;10(2):229–241. doi: 10.20411/pai.v10i2.873
10.20411/pai.v10i2.873
Background: Endophthalmitis, a severe infection of the intraocular tissues that can result in permanent loss of vision if not immediately treated, is often caused by fungi, namely Candida albicans. Treatment options are limited due to a lack of ocular penetration of antifungal drugs. Rezafungin, an echinocandin antifungal with a long half-life, which was recently approved by the US Food and Drug Administration (FDA), has shown efficacy against candidiasis.
Methods: In this study, using a rabbit model, we compared rezafungin, micafungin, and voriconazole in a hematogenous C. albicans endophthalmitis rabbit model. Fungal burden was determined in the aqueous humor, vitreous humor, choroid-retina, and the kidneys of infected rabbits; eye lesions were visualized by indirect ophthalmoscopy.
Results: No fungal growth was detected in the aqueous humor, vitreous humor, or choroid-retina of rabbits treated with 10 mg/kg rezafungin at the time of fungal inoculation. Additionally, rabbits given 10 mg/kg rezafungin showed the lowest kidney fungal burden (average log colony-forming units [CFUs]/g of < 0.5). In contrast, animals given either micafungin (6.2 mg/kg) or voriconazole (10 mg/kg) in the same treatment regimen were positive for fungal infection as measured by CFUs in each of these areas, demonstrating fungal burden. Additionally, significant increases in eye lesion scores were observed in rabbits given either micafungin or voriconazole, while no eye lesions were noted in rabbits that received rezafungin.
Conclusion: Taken together, these results indicate that rezafungin was effective at reducing the acute fungal burden and subsequent eye lesions caused by C. albicans-induced endophthalmitis.
Rezafungin; Candida albicans; Fungal Endophthalmitis; Echinocandin; Ocular Fungal Infection; Invasive Candidiasis
Endophthalmitis is a severe infection of the intraocular tissues that threatens both the aqueous and vitreous humor. This infection ultimately results in inflammation that can damage an individual’s vision, particularly in immunocompromised patients, resulting in permanent and devastating loss of vision if not treated within days of initial infection [1–3]. The nidus of infection for endophthalmitis is typically exogenous infection, postoperative or post-trauma; however, endogenous (an infection caused by microbes already present in the body) endophthalmitis has also become increasingly relevant as it often occurs as a complication of disseminated candidiasis [4, 5].
While both bacterial and fungal organisms can be causative of endophthalmitis, Candida species are of particular relevance as they have been identified on contaminated medical tools in cases of exogenous infection, and are implicated in hematogenous spread in endogenous cases [6]. Fungal endophthalmitis is of specific clinical significance due to the potential for hematogenous spread, as well as the possibility of systemic spread, which may involve other organs in the process [7]. C. albicans accounts for 60% to 65% of fungal endophthalmitis cases [1], making this species of great importance in diagnosis and treatment of fungal endophthalmitis. Vitrectomy, a surgical procedure where the vitreous humor gel is removed, has been used as treatment to improve visual acuity; however, it is rather invasive [8]. Studies attempting to use corticosteroids to reduce inflammation in vivo have been unreliable [2]. More importantly, despite the availability of antifungal agents, effective treatment against endophthalmitis remains challenging due to limited ocular penetration of certain drugs.
Penetration issues have been highlighted in studies evaluating the efficacy of caspofungin, fluconazole, and voriconazole, all of which have failed to effectively treat fungal endophthalmitis caused by Candida, citing difficulty in their delivery to the eye [9–11]. Thus, there is an unmet need to successfully treat this devastating disease. Echinocandins, including caspofungin, micafungin, and anidulafungin, have been reported to exhibit poor penetration into ocular compartments due to their large molecular weight and high protein binding, rendering them suboptimal for treating fungal endophthalmitis despite being first-line therapy for candidemia [12, 13]. In contrast, azoles such as fluconazole and voriconazole achieve therapeutic levels in the aqueous and vitreous humor and are frequently used in the treatment of intraocular fungal infections [14]. Voriconazole in particular is noted for its broad-spectrum activity against Candida spp., including many non-albicans species and molds such as Aspergillus [15]. Flucytosine also achieves high intraocular concentrations due to its low molecular weight and minimal protein binding, making it a valuable adjunct in certain clinical scenarios [16].
Newly published 2025 European Confederation of Medical Mycology (ECMM), International Society for Human and Animal Mycology (ISHAM), and American Society for Microbiology (ASM) (ECMM–ISHAM–ASM) global guideline for invasive candidiasis [17], recommend azole agents—specifically fluconazole and voriconazole—as firstline systemic therapy for ocular Candida infections, including chorioretinitis and endophthalmitis, provided the isolate is susceptible. Liposomal amphotericin B (LAmB), at 3–5 mg/kg daily, is recommended as the primary alternative when azoles are contraindicated, ineffective, or in resistant strains, and when susceptibility is unknown. At present, echinocandins are not recommended for routine ocular candidiasis therapy, except potentially in early-stage chorioretinitis lacking vitreal involvement, where combination therapy may be considered as adjunctive treatment.
Rezafungin, a novel semisynthetic echinocandin with an extended half-life, has shown promise in treating invasive candidiasis with less frequent dosing compared to traditional antifungals [18]. Rezafungin was recently approved (2023) for use in adults with invasive Candida infection, in the EU, UK, and the United States [19]. As defined by cure rates and mortality, rezafungin has been shown to possess non-inferior efficacy to first-generation echinocandins such as caspofungin and is approved to be taken on a weekly rather than a daily basis, which increases the advantage of drug exposure. Previous studies of fungal endophthalmitis using rabbit models have investigated micafungin and caspofungin and have established starting points for effective concentrations [20]. However, the efficacy of rezafungin for fungal endophthalmitis has not yet been examined.
In this study, we evaluated rezafungin compared to voriconazole or micafungin in a rabbit endophthalmitis model of hematogenous C. albicans infection. The antifungal activity of rezafungin was assessed by measuring fungal burden in ocular tissues and evaluating clinical lesions and severity in the eyes. The effect of rezafungin on kidney tissue burden was also evaluated.
Initially, MIC of the infecting strain (C. albicans SC5314 isolated from a clinical invasive candidiasis sample) against rezafungin, micafungin, and voriconazole was conducted following the Clinical and Laboratory Standards Institute (CLSI) document M27-Ed4, 2017 [21]. The goal of this MIC test was to confirm whether the strain was susceptible to rezafungin and comparators (Table 1).
Table 1. Susceptibility Data for the Test Compounds Against the Infecting C. albicans SC5314 Strain (µg/mL)
|
Test Compound |
C. albicans SC5314 |
|
Rezafungin |
0.016 |
|
Micafungin |
≤0.016 |
|
Voriconazole |
0.008 |
Animal experiments were performed upon review and approval of a protocol by the Case Western Reserve University Institutional Animal Care and Use Committee (protocol number 2018-0083). All procedures in the protocol followed the Animal Welfare Act, the Guide for the Care and Use of Laboratory Animals, and the Office of Laboratory Animal Welfare.
Female New Zealand White rabbits were obtained from Covance, Inc., with a body weight of 2.5–3.5 kg. Animals were allowed to acclimate for a minimum of 7 days prior to conducting the experiment. Environmental controls for the animal room were set to maintain a temperature of 16–22 °C, a relative humidity of 30% to 70%, and a 12h:12h light-dark cycle.
Rabbits were pre-sedated with acepromazine and butorphanol and anesthetized with isoflurane prior to inoculation. Silicone catheters were placed into the jugular vein and tunneled subcutaneously as described previously [22]. Forty-eight hours after catheter implantation, the animals were injected intravenously (IV) with 5 × 106 colony-forming units (CFUs) of C. albicans SC5314, resuspended in 2 mL of phosphate-buffered saline (PBS), via the catheter. Animals were divided into 4 groups: 1) rezafungin 10 mg/kg (200 mg human equivalent), 2) micafungin 6.2 mg/kg (100 mg human equivalent), 3) voriconazole 10 mg/kg (133 mg human equivalent), and 4) vehicle-treated control group. The test compounds were prepared in accordance with the manufacturer’s instructions. Specifically, VFEND Vials containing 200 mg lyophilized voriconazole were reconstituted with sterile water for injection to produce a solution containing 10 mg/mL. Based on the weight of the animals, the appropriate concentration of stock was further diluted in sterile water for injection to a final volume of 6 mL. Mycamine 50 (micafungin) mg vials were reconstituted with 5 ml of 0.9% saline and protected from light. Based on the weight of the animals, the appropriate concentration of stock was further diluted in 5% dextrose injection to a final volume of 6 mL. Rezafungin powder was reconstituted in a stock solution of 2 mL 0.9% saline for injection. Based on the weight of the animals, the appropriate concentration of stock was further diluted in 0.9% saline for injection to a final volume of 6 mL. All compounds were administered at 2 mL increments every 30 minutes for 2 hours via slow IV bolus via catheter at 0 and 80 hours post challenge; animals were followed for up to 8 days post inoculation. Each treatment group was allotted 6 animals. However, several animals were removed from the study due to catheter failure. Therefore, the rezafungin-, micafungin-, voriconazole-, and vehicle–treated groups had 6, 5, 4, and 5 rabbits, respectively.
Immediately prior to euthanasia at day 8, the rabbits were anesthetized and their eyes examined for lesions. Both eyes were dilated with drops of 1% Atropine Sulfate Ophthalmic Solution (Akorn, Inc.) and 0.5% Tropicamide Ophthalmic Solution (Akorn, Inc.), and eye lesions were visualized by indirect ophthalmoscopy. Research staff were trained by third year ophthalmology resident Gustavo Munguba, MD, PhD, on proper methods for dilating and evaluating the fungal lesions in the eye. An ION Vision eZView 20D Lens was used during the examinations. Each eye was evaluated independently using a standard numerical score based on the number and size of the lesions as well as the total ocular damage caused by infection of the eye. The severity of each lesion was scored on a scale of 1+ to 4+, as follows: 1+, lesion barely visible; 2+, lesion small but easily visible; 3+, lesion large but less than 1 disk diameter in size; and 4+, lesion larger than 1 disk diameter. The total eye severity score was equal to the sum of the number of eye lesions multiplied by their severity scores [23]. The entire vitreous aqueous humor from each eye was collected, weighed, and homogenized.
The entire vitreous humor sample was plated onto Sabouraud dextrose agar (Hardy Diagnostics). Kidney and choroid-retina samples were also collected, weighed, homogenized, and serially diluted in normal saline (0.085%) for enumeration of tissue fungal burden, calculated based on average log CFUs/g for the tissue samples [24].
To compare values obtained from experiments related to fungal burden and clinical lesions, one-way ANOVA with a Bonferroni post-hoc test was employed in determining significance. All statistical analyses were performed using SPSS for Windows, version 29.0. A P-value of < 0.05 was considered statistically significant.
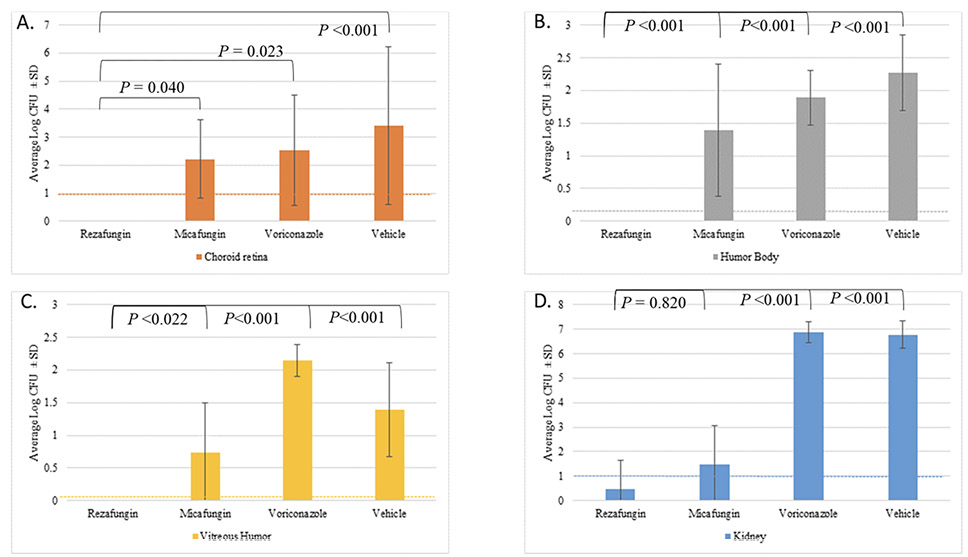
To evaluate the ability of the 3 antifungal treatments and vehicle-treated control to reduce fungal burden in ocular tissues, burden was assessed in key compartments of the eye. Tissue samples from the choroid retina, aqueous humor, vitreous humor, and kidneys were collected and analyzed for fungal burden. Figure 1 shows the average log CFUs /g ± SD for the tissue samples obtained.
In the choroid retina, the vehicle control group demonstrated the highest tissue fungal burden (average log CFUs/g ± SD of 3.42 ± 2.8). Animals given rezafungin had no detectable CFUs in either the right or left choroid retinas. In contrast, animals given micafungin or voriconazole showed fungal burdens of 2.21 ± 1.4 and 2.54 ± 2.0 log CFUs/g, respectively. Additionally, the rezafungin group showed significant antifungal activity when compared to the micafungin (P = 0.040), voriconazole (P=0.023), and vehicle control (P<0.001).
A similar trend was observed in the aqueous humor, where the vehicle control group exhibited the highest tissue fungal burden (2.27 ± 0.6 log CFUs/g). There were also no detectable CFUs in either the right or left humor bodies in animals given rezafungin. Animals given micafungin and voriconazole showed a fungal burden of 1.39 ± 1.0 and 1.89 ± 0.4 log CFUs/g, respectively. The rezafungin group showed significant antifungal activity when compared to the micafungin, voriconazole, and vehicle control groups (P<0.001).
The vehicle control group demonstrated a tissue fungal burden of 1.39 ± 0.7 CFUs/g ± SD in the vitreous humor. Similar to the other ocular compartments, animals given rezafungin had no detectable CFUs in either the right or left vitreous humors. In comparison, animals given micafungin and voriconazole showed a fungal burden of 0.74 ± 0.8 and 2.14 ± 0.2 log CFUs/g, respectively. The rezafungin group showed significant antifungal activity when compared to the micafungin (P=0.022), voriconazole (P<0.001), and vehicle control groups (P<0.001).
Fungal burden in the kidneys was evaluated to provide insight into the overall systemic efficacy of the antifungals. In the kidneys, the voriconazole and vehicle groups demonstrated the highest tissue fungal burden with 6.88 ± 0.4 log CFUs/g and 6.77 ± 0.6 log CFUs/g, respectively. Animals given rezafungin showed the lowest tissue fungal burden (0.47 ± 1.2 CFUs/g). The micafungin group had an average of 1.50 ± 1.4 log CFUs/g. The rezafungin and micafungin- groups demonstrated a significant reduction in tissue fungal burden when compared to the vehicle control (P<0.001). There was no significant difference between the rezafungin and micafungin groups (P=0.820).

Figure 1. Tissue fungal burden for the kidney, choroid retina, aqueous humor, and vitreous humora.
aVitreous humor sample was collected in its entirety and plated directly.
The limit of detection (LOD) for the ---- kidney and ---- choroid retina were both 10 CFUs, while the LOD for the ---- aqueous humor and ---- vitreous humor samples were 1 and 2 CFUs, respectively.
Table 2 shows the effects of treatment on the severity of Candida endophthalmitis as determined by indirect ophthalmoscopy scoring. Furthermore, Figure 2 shows representative photographs of indirect ophthalmoscopy done for: (A) rezafungin and (B) vehicle. As shown, the eye from animals administered rezafungin demonstrates a visible fundus (inner, back surface of the eyeball, opposite to the lens), healthy optic disc, and blood vessels with no visible abnormalities. In contrast, the fundus of the animals given vehicle alone could not be visualized due to vitreal haze; additionally, there are 2 yellow-white fluffy lesions indicating Candida infection. The vehicle control group demonstrated the highest eye severity scores with an average ± SD of 3.2 ± 2.5. No lesions were observed in the rezafungin group (average eye severity score of 0 ± 0). While animals given micafungin and voriconazole showed average eye severity scores ± SD of 1.9 ± 1.4 and 2.5 ± 1.8, respectively. The rezafungin group showed significant antifungal activity when compared to the voriconazole (P=0.007) and vehicle control groups (P<0.001). There was no significant difference between the rezafungin and micafungin groups.
Table 2. Effects of Treatments on the Severity of Candida endophthalmitis as Determined by Indirect Ophthalmoscopy Scoring
|
Treatment Group |
Average Eye Severity Score ± SD |
|
Rezafungin |
0 ± 0a,b |
|
Micafungin |
1.9 ± 1.4 |
|
Voriconazole |
2.5 ± 1.8 |
|
Vehicle Control |
3.2 ± 2.5 |
a P<0.001 when compared to the vehicle control
bP=0.007 when compared to the voriconazole-treated group.
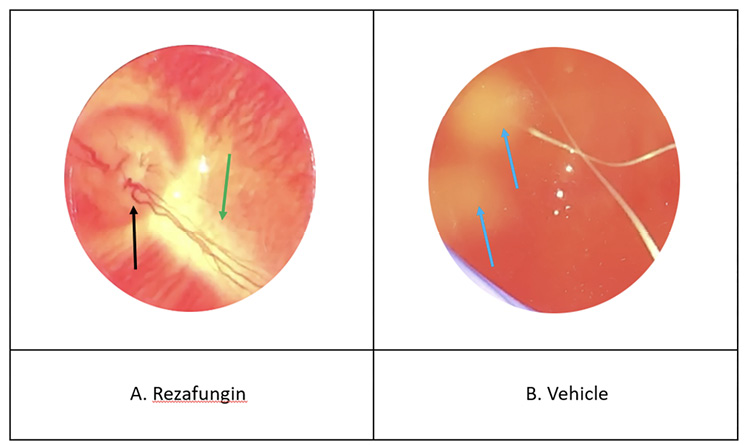
Figure 2. Representative photographs of the indirect ophthalmoscopy.
Representative photographs of indirect ophthalmoscopy done for the (A) rezafungin and (B) vehicle -treated groups. As can be seen, the rezafungin–treated eye demonstrates a visible fundus (→), healthy optic disc (→), and blood vessels (→) with no visible abnormalities. In contrast, the fundus of the vehicle–treated eye could not be visualized due to vitreal haze; additionally, there are 2 yellow-white fluffy lesions (→) indicating Candida infection.
Fungal endophthalmitis is challenging to diagnose and treat, as the infection can rapidly progress and lead to irreversible vision loss without effective intervention. Because intraocular Candida infections are often linked to systemic infections, systemic antifungal therapy is often reported, although agents need to cross the blood-retina barrier and achieve high ocular concentrations [25]. While intravitreal antifungal therapy such as voriconazole or amphotericin B can be effective, it often requires multiple injections and carries risks such as retinal toxicity [26]. Additionally, studies have attempted to address fungal endophthalmitis with drugs such as isavuconazole; however, these studies focused on Aspergillus species and require further investigation regarding their efficacy against candidal endophthalmitis [27]. Thus, there is a need to explore alternative strategies for addressing fungal endophthalmitis. In this study, we evaluated the antifungal activity of rezafungin on endophthalmitis caused by systemic C. albicans infection and quantified fungal burden in ocular tissues and kidneys, as well as assessing clinical lesion severity in a rabbit model, using voriconazole and micafungin as comparators.
The current data demonstrate that rezafungin was more effective than micafungin and voriconazole in reducing endophthalmitis in a rabbit model of C. albicans infection. As shown using eye fungal burden, as well as indirect ophthalmoscopy, rezafungin demonstrated significant antifungal activity when compared with animals administered either voriconazole or micafungin. No CFUs were detected in the aqueous humor, vitreous humor, or choroid-retina in the rabbits given rezafungin, and eye lesions were the lowest in the rezafungin groups, as there were no lesions observed. Since endophthalmitis associated with Candida infection is largely endogenous and thereby requires systemic therapy, it is important to note that rabbits in the rezafungin group demonstrated the lowest kidney fungal burden as well, demonstrating that this antifungal had broad target activity covering both eye infection as well as disseminated candidiasis.
The significant advantages that rezafungin demonstrates over its counterparts suggest that this therapeutic may have success in treating fungal endophthalmitis in other animal models, as well as more clinical success in patients. Not only does the reduction of fungal burden and prevention of ocular damage support this, but its increased half-life allows for prolonged sustainability of the drug level and gives rezafungin an advantage compared to other echinocandins. The increased half-life is attributed to the chemical structure of the compound, wherein a choline-derived moiety replaces the ornithine-derived hemiaminal structure seen in other echinocandins, leading to a more stable molecule [28]. The extended ability for rezafungin to be administered on a weekly basis rather than daily makes its therapeutic use far more practical [29].
Our study supports that rezafungin likely has sustained penetration into infected tissues. For example, in models of a liver abscess, rezafungin and micafungin have had their homogenous distribution compared, with rezafungin having more distribution in a shorter time frame, showing it can effectively distribute throughout infected tissue [30]. The overall pharmacokinetic profile of rezafungin includes its ability to inhibit the enzyme 1,3-β-D-glucan synthase, which helps to produce 1,3-β-D-glucan. This molecule is a crucial part of the cell wall of fungi, as without proper cell wall formation, there exists potential for osmotic instability and ultimately the death of the cell [26]. Micafungin functions similarly but lacks the structural stability and thus the extended half-life afforded by rezafungin [29]. Conversely, voriconazole is a triazole. Its mechanism involves interfering with ergosterol synthesis, a fungal membrane lipid [31]. This mechanism is less specific compared to rezafungin, and thus limits rather than stops cell growth of the fungi, as supported by the results of our study. Rezafungin is generally well tolerated in humans, with mild gastrointestinal and infusion-related reactions most frequently reported. Micafungin can rarely cause hepatotoxicity and hypersensitivity reactions. Voriconazole is associated with visual disturbances, hepatotoxicity, and phototoxicity
Future studies comparing the effect of rezafungin given twice a week versus voriconazole and micafungin given daily, are warranted as the difference in dosing between these agents is a variable in the current approach. In the current schema, rezafungin and the comparators were administered at the same time points (0- and 80-hours post inoculation), which favored rezafungin due to its longer half-life. However, future studies using rezafungin and comparators dosed daily are warranted, as well as comparative studies that use the approved daily dosing of micafungin and voriconazole, as well as additional comparators such as fluconazole, which achieves high concentrations in choroid/vitreous/retina [17]. Additionally, because this study was conducted in a rabbit model, additional studies validating the use of rezafungin in human clinical trials is necessary. Rezafungin can also be tested in combination with other antifungals to determine if a more effective treatment regimen is possible or whether antagonistic effects occur.
This study showed that rezafungin possesses superior antifungal activity compared to voriconazole and micafungin in this rabbit model of C. albicans endophthalmitis, effectively reducing the fungal burden in ocular tissues and preventing lesion formation, as well as reducing kidney fungal burden. Given the challenges associated with current antifungal treatments, including the need for frequent dosing, limited ocular penetration, and potential toxicity, the extended dosing interval and enhanced tissue distribution observed for rezafungin make it a promising candidate for further clinical investigation, including studies designed to address treatment versus prophylaxis. Future studies should explore its efficacy in the clinical setting and evaluate its potential to reduce the need for invasive interventions such as vitrectomy and ultimately improve treatment outcomes for patients with fungal endophthalmitis.
The authors wish to acknowledge and thank Dr. Gustavo Munguba, MD, PhD, for training in proper methods for dilating and evaluating the fungal lesions in the eye.
Rezafungin (formerly CD101) was provided by Cidara Therapeutics, and the study was cofunded by Cidara Therapeutics.
Dr. Mahmoud Ghannoum serves as a Senior Editor for Pathogens and Immunity, and is one of the co-founders of Next Trillion Sciences. The other authors report no other competing financial interests.
1. Asao K, Hashida N, Maruyama K, Motooka D, Nakamura S, Nishida K. Cases of endophthalmitis caused by Candida albicans and Candida dubliniensis identified via internal transcribed spacer deep sequencing. BMC Ophthalmol. 2024;24(1):444. doi: 10.1186/s12886-024-03702-4. PubMed PMID: 39385149; PMCID: PMC11463106.
2. Azhari J, Tetelbom PS, Sallam AB. The Role of Adjuvant Systemic and Intravitreal Corticosteroids in Fungal Endophthalmitis Treatment. J Fungi (Basel). 2023;9(12). doi: 10.3390/jof9121147. PubMed PMID: 38132748; PMCID: PMC10744273.
3. Durand ML. Bacterial and Fungal Endophthalmitis. Clin Microbiol Rev. 2017;30(3):597-613. doi: 10.1128/CMR.00113-16. PubMed PMID: 28356323; PMCID: PMC5475221.
4. Haseeb AA, Elhusseiny AM, Siddiqui MZ, Ahmad KT, Sallam AB. Fungal Endophthalmitis: A Comprehensive Review. J Fungi (Basel). 2021;7(11). doi: 10.3390/jof7110996. PubMed PMID: 34829283; PMCID: PMC8623405.
5. Rajagopal Padma M, Dinesh P, Sundaresan R, Athreya S, Shiju S, Maroor PS, Lalitha Hande R, Akhtar J, Chandra T, Ravi D, Lobo E, Ana Y, Shriyan P, Desai A, Rangaiah A, Munivenkatappa A, Krishna S, Basawarajappa SG, Sreedhara HG, ..., Rathnaiah Babu G. Corrigendum to “Second round statewide sentinel-based population survey for estimation of the burden of active infection and anti-SARS-CoV-2 IgG antibodies in the general population of Karnataka, India, during January-February 2021” [IJID Regions Vol 1(2021) pages 107-116]. IJID Reg. 2024;10:150. doi: 10.1016/j.ijregi.2024.01.001. PubMed PMID: 38314395; PMCID: PMC10835276.
6. Kim SW, Kim JH, Choi M, Lee SJ, Shin JP, Kim JG, Kang SW, Park KH, Korean Retina Society m. An Outbreak of Fungal Endophthalmitis After Cataract Surgery in South Korea. JAMA Ophthalmol. 2023;141(3):226-33. doi: 10.1001/jamaophthalmol.2022.5927. PubMed PMID: 36656597; PMCID: PMC9857837 Novartis, Bayer, Alcon, Roche, and Celltrion and owning stock in Oculight and RetiMark outside the submitted work. No other disclosures were reported.
7. Ramirez-Soto MC, Bonifaz A. Ocular Fungal Infections. J Fungi (Basel). 2022;8(10). doi: 10.3390/jof8101078. PubMed PMID: 36294643; PMCID: PMC9605517.
8. Negretti GS, Chan W, Pavesio C, Muqit MMK. Vitrectomy for endophthalmitis: 5-year study of outcomes and complications. BMJ Open Ophthalmol. 2020;5(1):e000423. doi: 10.1136/bmjophth-2019-000423. PubMed PMID: 32258421; PMCID: PMC7103804.
9. Fan N, Duan X, Liu X, Fan P, Chen N, Sun J. First Documented Successful Treatment of Chronic Postoperative Fungal Endophthalmitis Induced by Trichosporon Inkin with Fluconazole. Infect Drug Resist. 2024;17:5803-13. doi: 10.2147/IDR.S485152. PubMed PMID: 39734738; PMCID: PMC11681906.
10. Gauthier GM, Nork TM, Prince R, Andes D. Subtherapeutic ocular penetration of caspofungin and associated treatment failure in Candida albicans endophthalmitis. Clin Infect Dis. 2005;41(3):e27-8. doi: 10.1086/431761. PubMed PMID: 16007519.
11. Matsuo T, Kobayashi Y, Nishimura S, Yoshioka N, Takahashi Y, Iguchi Y. Intravitreal Fluconazole Injection for Fungal Endophthalmitis as Treatment Option in a Patient With End-Stage Liver and Kidney Diseases. J Med Cases. 2024;15(11):359-66. doi: 10.14740/jmc4302. PubMed PMID: 39421221; PMCID: PMC11483145.
12. Breit SM, Hariprasad SM, Mieler WF, Shah GK, Mills MD, Grand MG. Management of endogenous fungal endophthalmitis with voriconazole and caspofungin. Am J Ophthalmol. 2005;139(1):135-40. doi: 10.1016/j.ajo.2004.08.077. PubMed PMID: 15652837.
13. Ostrosky-Zeichner L, Pappas PG, Shoham S, Reboli A, Barron MA, Sims C, Wood C, Sobel JD. Improvement of a clinical prediction rule for clinical trials on prophylaxis for invasive candidiasis in the intensive care unit. Mycoses. 2011;54(1):46-51. doi: 10.1111/j.1439-0507.2009.01756.x. PubMed PMID: 19627509.
14. Hariprasad SM, Mieler WF, Holz ER, Gao H, Kim JE, Chi J, Prince RA. Determination of vitreous, aqueous, and plasma concentration of orally administered voriconazole in humans. Arch Ophthalmol. 2004;122(1):42-7. doi: 10.1001/archopht.122.1.42. PubMed PMID: 14718293.
15. Pappas G, Ierodiakonou V, Falagas ME. Lost in translation: differences in antimicrobial indication approval policies between the United States and Europe. Clin Ther. 2009;31(7):1595-603. doi: 10.1016/j.clinthera.2009.06.016. PubMed PMID: 19695409.
16. Ruhnke M, Bohme A, Buchheidt D, Cornely O, Donhuijsen K, Einsele H, Enzensberger R, Hebart H, Heussel CP, Horger M, Hof H, Karthaus M, Kruger W, Maschmeyer G, Penack O, Ritter J, Schwartz S, Infectious Diseases Working Party in H, Oncology of the German Society for H, Oncology. Diagnosis of invasive fungal infections in hematology and oncology--guidelines from the Infectious Diseases Working Party in Haematology and Oncology of the German Society for Haematology and Oncology (AGIHO). Ann Oncol. 2012;23(4):823-33. doi: 10.1093/annonc/mdr407. PubMed PMID: 21948809.
17. Cornely OA, Sprute R, Bassetti M, Chen SC, Groll AH, Kurzai O, Lass-Florl C, Ostrosky-Zeichner L, Rautemaa-Richardson R, Revathi G, Santolaya ME, White PL, Alastruey-Izquierdo A, Arendrup MC, Baddley J, Barac A, Ben-Ami R, Brink AJ, Grothe JH, Guinea J, Hagen F, Hochhegger B, Hoenigl M, Husain S, Jabeen K, Jensen HE, Kanj SS, Koehler P, Lehrnbecher T, Lewis RE, Meis JF, Nguyen MH, Pana ZD, Rath PM, Reinhold I, Seidel D, Takazono T, Vinh DC, Zhang SX, Afeltra J, Al-Hatmi AMS, Arastehfar A, Arikan-Akdagli S, Bongomin F, Carlesse F, Chayakulkeeree M, Chai LYA, Chamani-Tabriz L, Chiller T, Chowdhary A, Clancy CJ, Colombo AL, Cortegiani A, Corzo Leon DE, Drgona L, Dudakova A, Farooqi J, Gago S, Ilkit M, Jenks JD, Klimko N, Krause R, Kumar A, Lagrou K, Lionakis MS, Lmimouni BE, Mansour MK, Meletiadis J, Mellinghoff SC, Mer M, Mikulska M, Montravers P, Neoh CF, Ozenci V, Pagano L, Pappas P, Patterson TF, Puerta-Alcalde P, Rahimli L, Rahn S, Roilides E, Rotstein C, Ruegamer T, Sabino R, Salmanton-Garcia J, Schwartz IS, Segal E, Sidharthan N, Singhal T, Sinko J, Soman R, Spec A, Steinmann J, Stemler J, Taj-Aldeen SJ, Talento AF, Thompson GR, 3rd, Toebben C, Villanueva-Lozano H, Wahyuningsih R, Weinbergerova B, Wiederhold N, Willinger B, Woo PCY, Zhu LP. Global guideline for the diagnosis and management of candidiasis: an initiative of the ECMM in cooperation with ISHAM and ASM. Lancet Infect Dis. 2025;25(5):e280-e93. doi: 10.1016/S1473-3099(24)00749-7. PubMed PMID: 39956121.
18. Ham YY, Lewis JS, 2nd, Thompson GR, 3rd. Rezafungin: a novel antifungal for the treatment of invasive candidiasis. Future Microbiol. 2021;16(1):27-36. doi: 10.2217/fmb-2020-0217. PubMed PMID: 33438477.
19. Fung S, Shirley M. Rezafungin: A Review in Invasive Candidiasis. Drugs. 2025;85(3):415-23. doi: 10.1007/s40265-024-02134-0. PubMed PMID: 39913021.
20. Kapur R, Kim B, Tu EY, Birnbaum A, Fiscella R, Navare S, Blair MP, Edward DP, Carroll J, Lim JI. The Safe and Non-Toxic Dose of Intravitreal Micafungin and Caspofungin in a Rabbit Model. Investigative Ophthalmology & Visual Science. 2010;51(13):3324-.
21. CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. CLSI standard M27. Wayne, PA: CLSI; 2017.
22. Schinabeck MK, Long LA, Hossain MA, Chandra J, Mukherjee PK, Mohamed S, Ghannoum MA. Rabbit model of Candida albicans biofilm infection: liposomal amphotericin B antifungal lock therapy. Antimicrob Agents Chemother. 2004;48(5):1727-32. doi: 10.1128/AAC.48.5.1727-1732.2004. PubMed PMID: 15105127; PMCID: PMC400590.
23. Louie A, Liu W, Miller DA, Sucke AC, Liu QF, Drusano GL, Mayers M, Miller MH. Efficacies of high-dose fluconazole plus amphotericin B and high-dose fluconazole plus 5-fluorocytosine versus amphotericin B, fluconazole, and 5-fluorocytosine monotherapies in treatment of experimental endocarditis, endophthalmitis, and pyelonephritis due to Candida albicans. Antimicrob Agents Chemother. 1999;43(12):2831-40. doi: 10.1128/AAC.43.12.2831. PubMed PMID: 10582868; PMCID: PMC89573.
24. Filler SG, Crislip MA, Mayer CL, Edwards JE, Jr. Comparison of fluconazole and amphotericin B for treatment of disseminated candidiasis and endophthalmitis in rabbits. Antimicrob Agents Chemother. 1991;35(2):288-92. doi: 10.1128/AAC.35.2.288. PubMed PMID: 2024963; PMCID: PMC244993.
25. Sallam A, Taylor SR, Khan A, McCluskey P, Lynn WA, Manku K, Pacheco PA, Lightman S. Factors determining visual outcome in endogenous Candida endophthalmitis. Retina. 2012;32(6):1129-34. doi: 10.1097/IAE.0b013e31822d3a34. PubMed PMID: 22298012.
26. Silva RA, Sridhar J, Miller D, Wykoff CC, Flynn HW, Jr. Exogenous fungal endophthalmitis: an analysis of isolates and susceptibilities to antifungal agents over a 20-year period (1990-2010). Am J Ophthalmol. 2015;159(2):257-64 e1. doi: 10.1016/j.ajo.2014.10.027. PubMed PMID: 25449001.
27. Guest JM, Singh PK, Revankar SG, Chandrasekar PH, Kumar A. Isavuconazole for Treatment of Experimental Fungal Endophthalmitis Caused by Aspergillus fumigatus. Antimicrob Agents Chemother. 2018;62(11). doi: 10.1128/AAC.01537-18. PubMed PMID: 30201814; PMCID: PMC6201095.
28. Wiederhold NP. Pharmacodynamics, Mechanisms of Action and Resistance, and Spectrum of Activity of New Antifungal Agents. J Fungi (Basel). 2022;8(8). doi: 10.3390/jof8080857. PubMed PMID: 36012845; PMCID: PMC9410397.
29. Forrister NM, McCarty TP, Pappas PG. New Perspectives on Antimicrobial Agents: Rezafungin. Antimicrob Agents Chemother. 2025;69(1):e0064623. doi: 10.1128/aac.00646-23. PubMed PMID: 39665557; PMCID: PMC11784067.
30. Zhao Y, Prideaux B, Baistrocchi S, Sheppard DC, Perlin DS. Beyond tissue concentrations: antifungal penetration at the site of infection. Med Mycol. 2019;57(Supplement_2):S161-S7. doi: 10.1093/mmy/myy067. PubMed PMID: 30816968; PMCID: PMC6506603.
31. Sanati H, Belanger P, Fratti R, Ghannoum M. A new triazole, voriconazole (UK-109,496), blocks sterol biosynthesis in Candida albicans and Candida krusei. Antimicrob Agents Chemother. 1997;41(11):2492-6. doi: 10.1128/AAC.41.11.2492. PubMed PMID: 9371355; PMCID: PMC164150.
Submitted July 25, 2025 | Accepted October 6, 2025 | Published October 21, 2025
Copyright © 2025 The Authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License.