Mariangela Scavone1#, Roberta Rovito2#, Claudia Ghali1, Antonella Fioretti1, Bianca Clerici2, Elena Bossi1, Camilla Tincati2, Andrea Santoro2, Elisa Borghi3, Gian Marco Podda1, Giulia Marchetti2
1Division of General Medicine II, Department of Health Sciences, ASST Santi Paolo e Carlo, University of Milan, Milan, Italy
2Clinic of Infectious Diseases and Tropical Medicine, Department of Health Sciences, ASST Santi Paolo e Carlo, University of Milan, Italy
3Clinical Microbiology, Department of Health Sciences, ASST Santi Paolo e Carlo, University of Milan, Italy
#These authors contributed equally to this manuscript
Gian Marco Podda
gmpodda@gmail.com
Scavone M, Rovito R, Ghali C, Fioretti A, Clerici B, Bossi E, Tincati C, Santoro A, Borghi E, Podda GM, Marchetti G. Investigating the Interplay of SARS-CoV-2 RNAemia and Peripheral Inflammation in Platelet Dysfunction During Acute SARS-CoV-2 Infection. Pathogens and Immunity. 2025;10(2):149–167. doi: 10.20411/pai.v10i2.823
10.20411/pai.v10i2.823
Background: Circulating degranulated platelets have been described during acute SARS-CoV-2 infection and associated with COVID-19 complications. This study investigated the relationship between the presence of plasma SARS-CoV-2 RNA (ie, SARS-CoV-2 RNAemia), systemic inflammation, and platelet dysfunction in a group of patients with COVID-19. Unlike our previous publication, which focused on platelet characterization, this work explores potential determinants of platelet activation, based on a distinct subset of patients with available stored samples.
Methods: Patients with COVID-19 were stratified by platelet δ-granule content using the luciferin/luciferase assay into 2 groups: normal (COVδ-norm) and low (COVδ-low). Plasma SARS-CoV-2 RNAemia (RT-qPCR), cytokines, and chemokines (Cytometric Bead Array) were quantified on plasma samples. Markers of platelet activation were measured by flow cytometry in whole blood.
Results: A total of 75 patients with COVID-19 were enrolled; 57 presented normal levels of platelet δ-granule content (COVδ-norm) and 18 had low levels of platelet δ-granules (COVδ-low). Groups were comparable in terms of age, sex, comorbidities, and SARS-CoV-2 RNAemia levels. Patients in the COVδ-low group showed significantly higher chemokine and cytokine levels compared to those in the COVδ-norm group, with strong correlations between IL-6, as well as granulocyte-macrophage colony-stimulating factor (GM-CSF), with platelet degranulation parameters. A similar trend, albeit less pronounced, was observed when patients were stratified based on their platelet activation phenotype.
Conclusions: These findings suggest that peripheral inflammation, rather than SARS-CoV-2 RNAemia, is associated with platelet dysfunction during acute SARS-CoV-2 infection.
COVID-19; Platelets Dysfunction; SARS-CoV-2 viral RNA; Chemokines and Cytokines; Inflammation
COVID-19 is characterized by a heterogeneous spectrum of clinical manifestations, ranging from flu-like symptoms to life-threatening multi-organ dysfunction [1]. The severe form of COVID-19, which occurs in approximately 14% of infected individuals [2], often leads to thrombotic and bleeding complications [3]. Since the beginning of the pandemic, there have been early post-mortem descriptions of platelet thrombi in the microcapillaries of the lung, heart, kidney, and skin of patients with COVID-19 [4]. Because of these findings, anticoagulation has been widely used in the management of inpatients with COVID-19 who are, however, also prone to bleeding [5]. One of the contributors for such a unique thrombotic and bleeding tendency might be the presence of activated and degranulated platelets in COVID-19 patients [6–9]. The mechanisms underlying the aberrant activation and degranulation of platelets in COVID-19, however, remain to be elucidated. SARS-CoV-2 RNA has been detected in various tissues during acute COVID-19, including the bloodstream [4, 10–14]. It is detectable as early as the first week of infection [15, 16] and is associated with inflammation, tissue damage, disease progression, and death [17–19]. Viral persistence may also contribute to long-COVID, although the exact mechanisms of viral dissemination remain unclear, with the bloodstream likely playing a key role [20]. SARS-CoV-2 RNA has been found in platelets of patients with COVID-19, often in the context of elevated pro-inflammatory cytokines and platelet hyperactivation [8, 21]. In some cases, the presence of replication-competent virus in platelets has been associated with fatal outcomes [22], suggesting that the virus may enter platelets during their formation in the bone marrow, similarly to other viral infections such as HIV, dengue, and influenza [23–25]. In addition, SARS-CoV-2 can interact directly with platelets [26] via the ACE2 receptor and other platelet receptors [27, 28]. During conditions such as sepsis or COVID-19, platelets are not merely innocent bystanders but key contributors of systemic inflammation [29, 30]. Unfortunately, the severe inflammation observed in COVID-19, driven by chemokines and cytokines, such as TNF, IL-6, and G-CSF [20, 31], further complicates the understanding of platelet dysfunction. Therefore, the aim of the present study was to investigate the interplay of SARS-CoV-2 RNAemia as well as peripheral inflammation with platelet dysfunction during acute COVID-19.
This study is based on a distinct subset of patients from the cohort described in our previous publication [9] with available stored plasma samples and complete data on δ-granule content and platelet activation markers. Unlike the previous analysis, which focused on platelet dysfunction per se, the current study investigates the pathophysiological relationship between SARS-CoV-2 RNAemia, systemic inflammation, and platelet dysfunction. The cohort consisted of individuals with SARS-CoV-2 infection, confirmed through RT-PCR on nasopharyngeal swab (ie, NP), enrolled between March 2021 and August 2022. Patients were hospitalized or referred to the outpatient clinic of the Department of Infectious Diseases and Tropical Medicine, University of Milan, ASST Santi Paolo e Carlo, Milan, due to their high risk of developing severe COVID-19. Patients on antiplatelet therapy or with pre-existing platelet dysfunctions were excluded from the study. The time of blood sampling since the onset of symptoms of acute SARS-CoV-2 infection was recorded for each study participant. The study was approved by the Institutional Ethics Committee (Comitato Etico ASST Santi Paolo e Carlo; 2020/ST/049, 2020/ST/049_BIS, 11/03/2020), and written informed consent was obtained from all study participants. All research was performed in accordance with the Declaration of Helsinki.
Each eligible patient underwent a single blood withdrawal for the purposes of the study. Venous blood samples were collected from the antecubital vein using a 21-gauge butterfly needle without applying a tourniquet to minimise platelet activation. The first 4 mL of blood were drawn into K2E EDTA tubes (Greiner) and analyzed for blood cell counts using a coulter haematology analyzer (Medonic M series 16). The remaining blood was anticoagulated with 109 mmol/L-1 trisodium citrate (9:1; vol:vol) for the assessment of platelet activation markers, such as platelet-monocyte aggregates and platelet nucleotide content, while K2E EDTA tubes were used for the quantification of plasmatic cytokines/chemokines and SARS-CoV-2 RT-qPCR analysis.
To evaluate platelet degranulation, intracellular levels of ADP and ATP were measured in platelet-rich plasma. These nucleotides are stored in dense granules and released upon platelet activation, leading to a marked decrease in intracellular ADP and a consequent increase in the ATP/ADP ratio. Platelet degranulation was assessed with the measurement of the content of platelet nucleotides, performed as previously described [9]. Briefly, a total of 0.5 mL of citrated platelet-rich plasma (PRP) was mixed with 0.05 mL EDTA (100 mM) and 450 µL absolute ethanol, followed by centrifugation at 16,000g for 45 minutes at 4 °C. The supernatant was then stored at -80 °C for further analysis. The total platelet content of ADP and ATP was measured using the firefly luciferin/luciferase method in a lumiaggregometer (Chrono-log V400) [32]. ADP and ATP levels were expressed as nmol/108 platelets, and the overall ATP/ADP ratio was calculated. Cut-off values for ADP and the ATP/ADP ratio were determined based on the 10th percentile (1.98 nmol/108 platelets) and 90th percentile (3.47 ratio), respectively, of a cohort of 36 healthy participants [9]. Platelet activation was indirectly assessed by quantifying circulating platelet-monocyte aggregates (PMAs), which form through the interaction between P-selectin on activated platelets and P-selectin glycoprotein ligand-1 on monocytes. As previously described, [9] 15 µL of citrated whole blood was stained with fluorescein isothiocyanate (FITC)-conjugated anti-human CD14 and allophycocyanin (APC)-conjugated anti-human CD41a for 20 minutes at room temperature in the dark. Samples were fixed, and erythrocytes were lysed by adding 0.5 mL of FACS Lyse Solution (BD Biosciences). PMAs were identified as double positive events for both platelet and monocyte markers (CD41a+ - CD14+). Data were analyzed using FACS Suite software (BD Biosciences), with results expressed as the percentage of positive cells. The cut-off value for PMAs was determined based on the 90th percentile of a cohort of 36 healthy subjects and was set at 23.02%.
Plasmatic cytokines (IFN-α, IFN-γ, IL-2, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12, IL-17A, and TNF-α) and the chemokine GM-CSF were quantified using the Human MACSPlex Cytokine 12 Kit (Miltenyi Biotec) according to the manufacturer’s instructions. Briefly, thawed plasma samples were diluted 1:4 with assay diluent and incubated with MACSPlex Cytokine 12 Capture Beads for 2 hours, followed by 1-hour incubation with the MACSPlex Cytokine 12 Detection reagent. Samples were resuspended in 0.3 mL of assay buffer, acquired on a FACS Verse Cytometer (BD Biosciences), and analyzed with FlowLogic v8 software (Inivai Technologies). A total of 7 individuals sampled before the pandemic era were included as controls. Samples were acquired with FACS Verse Cytometer (BD Biosciences), and data were analyzed with FlowLogic 7.3.
Viral RNA was extracted from 140 µL of thawed plasma by using the QIAamp Viral RNA Mini Kit (QIAGEN) and quantified by real-time PCR using the CDC 2019-nCoV_N1 primers and probe set (Centers for Disease Control and Prevention, Update June 2020) and the TaqPath™ 1-Step RT-qPCR Master Mix CG (ThermoFisher), which quantify the Nucleocapsid gene of SARS-CoV-2. The 2019-nCoV_N Positive Control plasmid (Integrated DNA Technologies, Inc.) was used for absolute quantification, while a non-template condition was used as negative control. The RPP30 quantification was employed for RNA extraction quality. The assay was performed in duplicate.
Eligible COVID-19 patients were categorized in 2 groups based on their platelet degranulation status, which was defined by the cut-off values of ADP and ATP/ADP (ADP content below 1.98 nmol/108 platelets and ATP/ADP ratio above 3.47, respectively). Continuous variables were compared using the Mann-Whitney U test or the unpaired t-test, according to data distribution, which was assessed using the D’Agostino Pearson test. Categorical variables were compared using Fisher’s exact test. Correlations between SARS-CoV-2 RNAemia or peripheral cytokine/chemokine levels and platelet markers were assessed using Pearson’s or Spearman’s correlation tests, according to data distribution. Data were analyzed and visualised using GraphPad Prism version 9.0 (GraphPad Software). Two-tailed P-values <0.05 were considered statistically significant.
A total of 75 patients with COVID-19 were included in the present study. The demographic and clinical characteristics of the study population are summarized in Table 1. Fifty-seven patients with COVID-19 were classified as having non-degranulated platelets (COVδ-norm) and 18 patients with COVID-19 as having degranulated platelets (COVδ-low). Age, sex, and comorbidities were comparable between the groups, with a median age of 66 years (IQR: 51-75) for COVδ-norm patients and 56 years (IQR: 38-75) for COVδ-low patients (P= 0.295) and a predominance of men in both groups (53% and 72%, respectively, P=0.143) (Table 1). Furthermore, patients in the COVδ-low group exhibited significantly higher neutrophil/lymphocyte ratios and significantly higher levels of C-reactive protein, D-dimer, and lactate dehydrogenase compared to the COVδ-norm group (Table 1). Significantly more patients in the COVδ-low group received low-molecular-weight heparin, were hospitalized, or died within 30 days, and none of them received SARS-CoV-2 antivirals (Table 1).
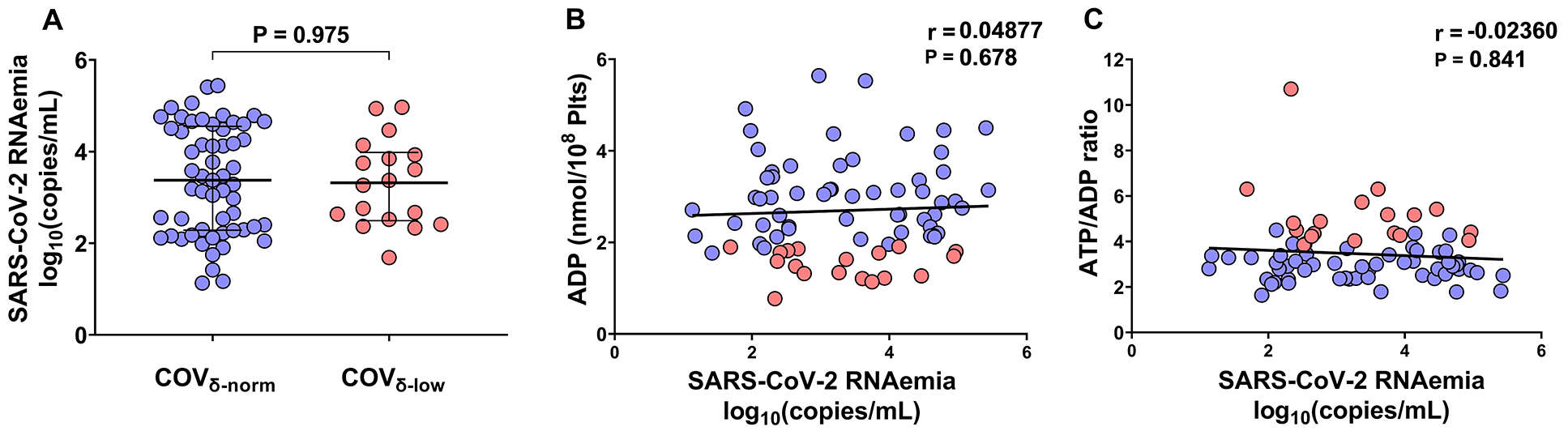
Firstly, we assessed SARS-CoV-2 RNAemia in the study population. There were no statistically significant differences in SARS-CoV-2 RNAemia between the COVδ-norm and COVδ-low groups [3.4 median log10(copies/mL), IQR 2.3 to 4.6 vs 3.3 median log10(copies/mL), IQR 2.5 to 4.0, respectively, P=0.9755] (Figure 1A). Furthermore, no statistically significant correlations were observed between the markers used to assess platelet degranulation and SARS-CoV-2 RNAemia, neither when considering the entire cohort (ADP: r=0.05, P=0.678; ATP/ADP ratio: r=-0.024, P=0.841) (Figure 1B-C) nor when patients were stratified based on their platelet degranulation status (COVδ-norm: ADP content r=0.078, P=0.565; ATP/ADP ratio: r=-0.025, P=0.856; COVδ-low: ADP content r=-0.046, P=0.855; ATP/ADP ratio: r=-0.168, P=0.504). These findings do not support an association of SARS-CoV-2 RNAemia with platelet degranulation.
Table 1. Demographic and Clinical Characteristics of the Study Patients
|
COVδ-norm |
COVδ-low |
P-value |
|
|
Clinical characteristics |
|||
|
Age (years), median (IQR) |
66 (51-75) |
56 (38-75) |
0.295 |
|
Sex, male, n (%) |
30 (53) |
13 (72) |
0.143 |
|
COVID-19 vaccine, n (%) |
38 (67) |
8 (44) |
0.092 |
|
Platelet parameters |
|||
|
Platelet δ-granules content: |
|||
|
ADP (nmol/108 plts), median (IQR) |
2.99 (2.34-3.54) |
1.61 (1.26-1.81) |
<0.001 |
|
ATP/ADP ratio, median (IQR) |
2.91 (2.41-3.30) |
4.66 (4.26-5.50) |
<0.001 |
|
Markers of platelet activation: |
|||
|
Platelet-monocytes aggregates, (%), median (IQR) |
21.9 (15.7-37.9) |
39.3 (18.9-67.8) |
0.029 |
|
Comorbidities, n (%) |
|||
|
Obesity |
12 (21) |
5 (28) |
0.552 |
|
Hypertension |
24 (42) |
8 (44) |
0.861 |
|
Diabetes |
4 (7) |
2 (11) |
0.579 |
|
Cardiovascular disease |
11 (19) |
4 (22) |
0.787 |
|
Chronic pulmonary disease |
10 (18) |
1 (6) |
0.210 |
|
Chronic kidney disease |
4 (7) |
2 (11) |
0.577 |
|
Mild liver disease |
2 (4) |
0 (0) |
0.421 |
|
Neurologic disease |
4 (7) |
1 (6) |
0.828 |
|
Psychiatric disorder |
3 (5) |
0 (0) |
0.321 |
|
Autoimmune disease |
4 (7) |
1 (6) |
0.828 |
|
Transplant |
2 (4) |
0 (0) |
0.421 |
|
Hematologic disorder |
4 (7) |
2 (11) |
0.577 |
|
Laboratory values, median (IQR) |
|||
|
Haemoglobin (g/dL) |
12.3 (11.6-14.0) |
12.7 (11.8-13.9) |
0.803 |
|
Haematocrit (%) |
37.7 (35.2-43.1) |
39.0 (36.5-41.6) |
0.784 |
|
Platelets (x103/mmc) |
209 (172-299) |
239 (162-305) |
0.597 |
|
WBC count (x103/mmc) |
5.9 (3.7-7.9) |
6.0 (5.3-8.5) |
0.586 |
|
Neutrophils (x103/mmc) |
3.4 (2.1-5.9) |
4.8 (4.1-7.1) |
0.071 |
|
Lymphocytes (x103/mmc) |
1.1 (0.8-1.8) |
0.8 (0.5-1.1) |
0.015 |
|
NL ratio |
2.8 (1.2-6.4) |
6.7 (3.6-11.4) |
0.003 |
|
C-reactive protein (mg/L) [n/N] |
23.4 (8.6-52.2)[54/57] |
47 (23.0-77.3) [18/18] |
0.040 |
|
Prothrombin time (INR) [n/N] |
1.1 (1.0-1.2) [55/57] |
(1.0-1.3) [18/18] |
0.837 |
|
D-dimer (ng/mL) [n/N] |
237 (0-332.8) [34/57] |
341 (286-487) [15/18] |
0.005 |
|
Lactate dehydrogenase (U/L) [n/N] |
221 (192-290) [53/57] |
301 (227-462) [18/18] |
0.033 |
|
PF ratio at admission [n/N] |
278 (208-311) [32/57] |
276 (165-327) [17/18] |
0.749 |
|
Treatments, n (%) |
|||
|
NSAID/COX-ib |
3 (5) |
3 (17) |
0.120 |
|
LMWH |
25 (44) |
14 (78) |
0.012 |
|
SARS-CoV-2 antivirals |
27 (47) |
0 (0) |
<0.001 |
|
Corticosteroids |
23 (2) |
12 (67) |
0.051 |
|
Tocilizumab |
1 (2) |
1 (6) |
0.383 |
|
Outcome, n (%) |
|||
|
Hospital admission |
32 (56) |
17 (94) |
0.003 |
|
Transfer to ICU |
3 (9) |
3 (18) |
0.120 |
|
Discharge |
29 (91) |
13 (76) |
0.112 |
|
Death |
0 (0) |
1 (6) |
0.073 |
|
Time admission to outcome (days), median (IQR) |
11 (5-25) |
14 (10-23) |
0.849 |
|
30-day mortality |
0 (0) |
3 (17) |
0.001 |
|
Complications, n (%) |
|||
|
Bleeding events |
1 (2) |
1 (6) |
0.383 |
|
Thrombotic events |
0 (0) |
1 (6) |
0.073 |
Legend. δ-norm, normal δ-granule content; δ-low, low δ-granule content; IQR, interquartile range; ADP, adenosine diphosphate; ATP, adenosine triphosphate; WBC, white blood cell; NL ratio, Neutrophil-Lymphocyte Ratio; PF-ratio, PaO2/FIO2 ratio; NSAID, non-steroidal anti-inflammatory drugs; COX-ib, COX-2 inhibitor; LMWH, Low-molecular-weight heparin; ICU, Intensive Care Unit. Statistical analyses, Mann-Whitney test, unpaired t-test, Fisher’s exact test, or Chi-square test, as appropriate. Two-tailed P-values <0.05 were deemed statistically significant (in bold).

Figure 1. Plasmatic SARS-CoV-2 RNAemia in patients with COVID-19 according to their platelet δ-granule content. (A) Plasmatic SARS-CoV-2 RNAemia in patients with COVID-19 with normal platelet δ-granule content (COVδ-norm, n=57) and low platelet δ-granule content (COVδ-low, n=18). (B) Correlations between SARS-CoV-2 RNAemia log10(copies/mL) with platelet ADP content (nmol/108 platelets) and (C) platelet ATP/ADP ratio. Median values with interquartile ranges (IQR) are shown for each group of patients. Data were analyzed using the Mann-Whitney test and the Spearman’s correlation test. Statistical significance was assumed for two-sided P-values <0.05.
Next, we evaluated the peripheral cytokine milieu in both patient groups. Significantly higher levels of GM-CSF, IFN-γ, TNF-α, IL-4, IL-5, IL-6, IL-10, IL-12, and IL-17A were observed in the COVδ-low group compared to the COVδ-norm group (Figure 2A), with the exception of IL-2 and IL-9, which were detectable only in 3 patients (data not shown). Median cytokine and chemokine values of 7 healthy participants sampled before the pandemic are included as reference (Figure 2A).
Since ADP is stored in platelet dense granules and released upon activation, intracellular ADP levels drop following degranulation. Meanwhile, ATP levels remain relatively stable, resulting in an increased ATP/ADP ratio. When evaluating the correlation between plasma cytokine and chemokine levels and ADP content across the entire patient cohort, a trend towards a negative correlation was observed, reaching statistical significance for GM-CSF (r=-0.52, P<0.001) (Figure 2B). Similar patterns were noted when stratifying patients based on their platelet degranulation status. The correlation between ADP and GM-CSF remained significant in both groups (COVδ-norm: r=-0.32, P=0.02; COVδ-low: r=-0.54, P=0.03) (Figure 2C-D). Statistical significance was also achieved, in the COVδ-norm group only, for IL-6 (r=-0.28, P=0.04) and IL-17α (COVδ-norm: r=-0.29, P=0.03) (Figure 2C). The positive correlation trend that we observed between plasma cytokine and chemokine levels with the ATP/ADP ratio in the overall cohort appeared to be mainly driven by the COVδ-low group (Figure 2D). Negative correlations between intracellular ADP levels and plasma cytokine concentrations, along with positive correlations between the ATP/ADP ratio and the same cytokines, indicate that greater platelet degranulation, reflected by lower ADP content and a higher ATP/ADP ratio, is associated with increased systemic inflammation. These findings suggest that plasma cytokine and chemokine levels are associated with platelet degranulation, with a stronger association in patients with more pronounced platelet degranulation. Indeed, whereas in the COVδ-norm group, only ADP appears to be negatively correlated with inflammation; in the COVδ-low group, both parameters used to assess platelet degranulation appear to be associated with inflammation. Specifically, ADP was negatively correlated with inflammation, and as expected, ATP/ADP positively correlated with inflammation.
Notably, whereas platelet degranulation appears to be associated with peripheral inflammation, in this group of individuals, SARS-CoV-2 RNAemia does not seem to be associated with inflammation, suggesting the involvement of other mechanisms (Figure 3).
Platelet activation and degranulation are distinct yet interconnected functional phases. Although activation typically precedes degranulation, it does not inevitably lead to it; platelets can become activated without releasing their granular contents. Given this functional dissociation, it is important to assess both processes independently. Having evaluated platelet degranulation and its association with systemic inflammation, we next focused on platelet activation per se to further explore its potential relationship with SARS-CoV-2 RNAemia and the peripheral cytokine milieu.
To investigate the relationship between platelet activation, SARS-CoV-2 RNAemia, and systemic inflammation, we stratified patients with COVID-19 into 2 groups based on PMAs. The cut-off for PMAs (23.02%) was derived from measurements in 36 healthy donors. Patients with PMA levels below this threshold were classified as COVPLT-norm (n=39), whereas those with PMA levels above the threshold were designated as COVPLT-act (n=35). SARS-CoV-2 RNAemia did not differ significantly between the 2 groups (COVPLT-norm: median 3.4 log₁₀[copies/mL], IQR 1.4–4.2; COVPLT-act: median 3.4 log₁₀[copies/mL], IQR 2.3–4.7; p=0.846) (Figure 4A).
Plasma cytokine and chemokine profiling revealed significantly higher concentrations of GM-CSF and IL-6 in the COVPLT-act group compared to COVPLT-norm (P=0.027 and P=0.002, respectively). Other cytokines, including TNF-α, IL-4, IL-10, and IL-17α, were modestly elevated in the COVPLT-act group but did not reach statistical significance. Similarly, IFN-γ, IL-5, and IL-12 levels did not differ significantly between the 2 groups (Figure 4B). Therefore, these data may suggest that peripheral inflammation is more strongly associated with degranulation than with activation, most likely due to the intrinsic functional significance of degranulation.
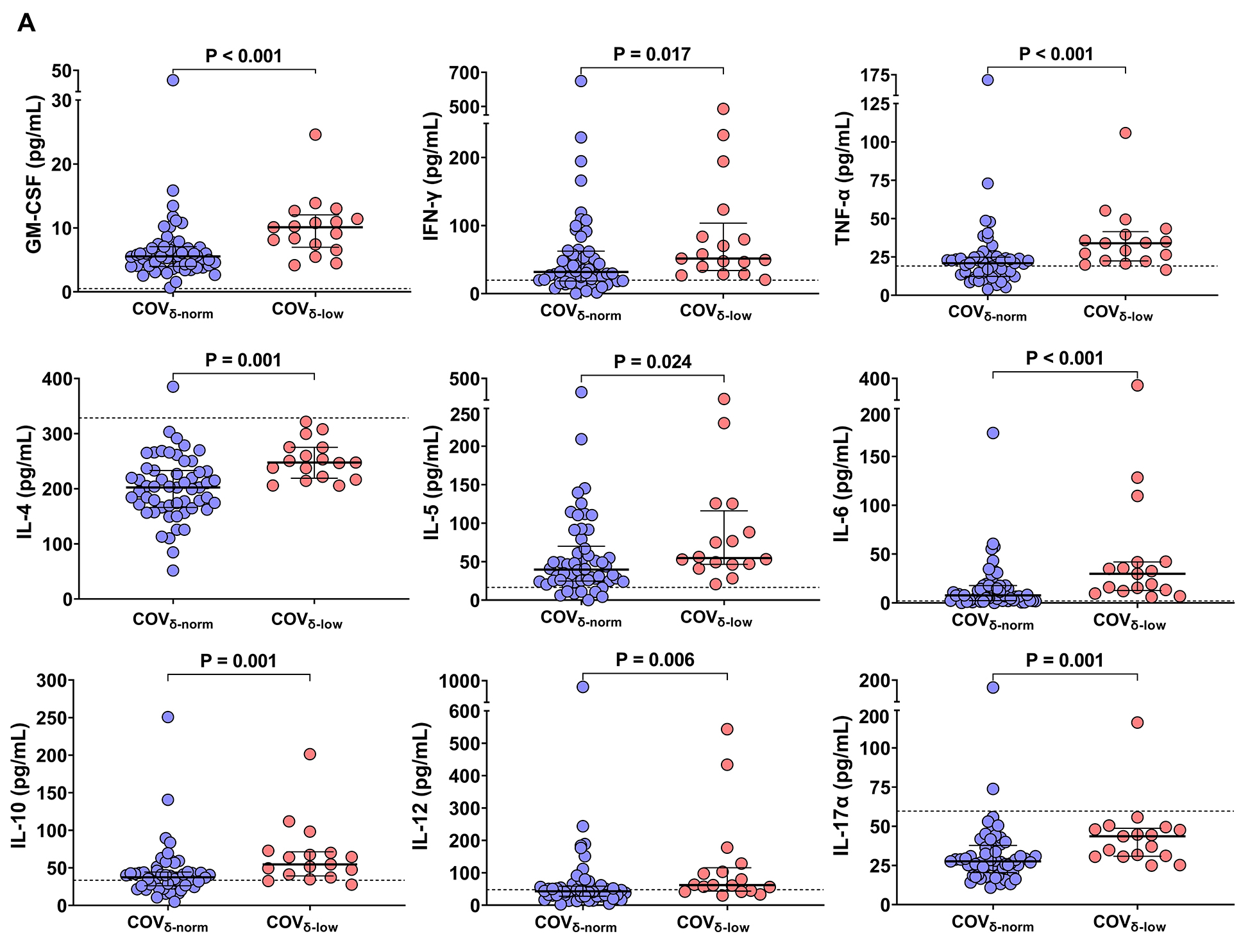
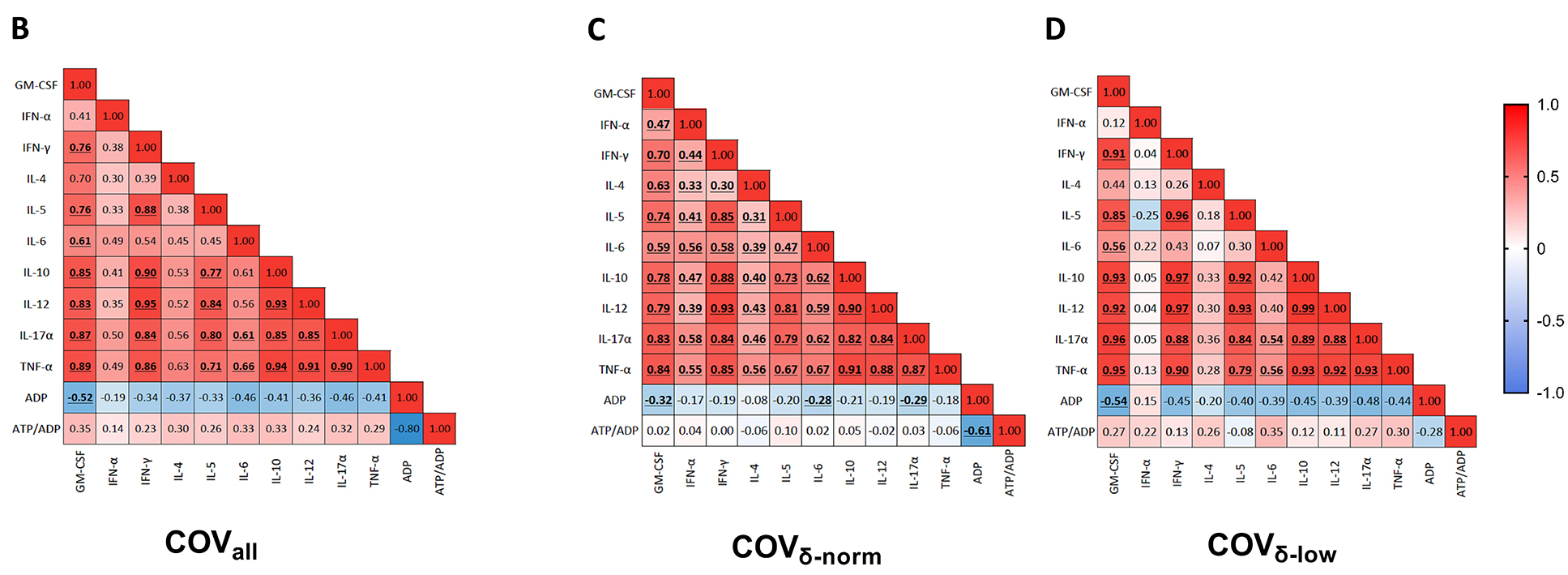
Figure 2. Plasmatic chemokine and cytokine levels in patients with COVID-19 according to their platelet δ-granule content. (A) Plasmatic chemokine and cytokine levels in patients with COVID-19 with normal platelet δ-granule content (COVδ-norm) and low platelet δ-granule content (COVδ-low). GM-CSF (n=55 and n=17), IFN-γ (n=53 and n=17), TNF-α (n=54 and n=17), IL-4 (n=55 and n=17), IL-5 (n=54 and n=16), IL-6 (n=53 and n=17), IL-10 (n=52 and n=17), IL-12 (n=53 and n=17), IL-17A (n=55 and n=17). Dots represent individual values. For each patient group, median values with interquartile ranges (IQR) are displayed. The dashed lines represent the median cytokine and chemokine value of 7 healthy subjects sampled before the pandemic. (B) Heatmap showing correlations between plasmatic chemokine and cytokine levels and platelet δ-granule content across the entire cohort of patients with COVID-19 , (C) only in COVδ-norm patients, and (D) only in COVδ-low patients. The color gradient represents the correlation coefficient (r); numerical values within each cell indicate the r value. Statistically significant correlations are highlighted in bold and underlined. Data were analyzed using the Mann–Whitney or t-test, as appropriate, and the Spearman’s correlation test. Statistical significance has been assumed for two-sided P-values <0.05.
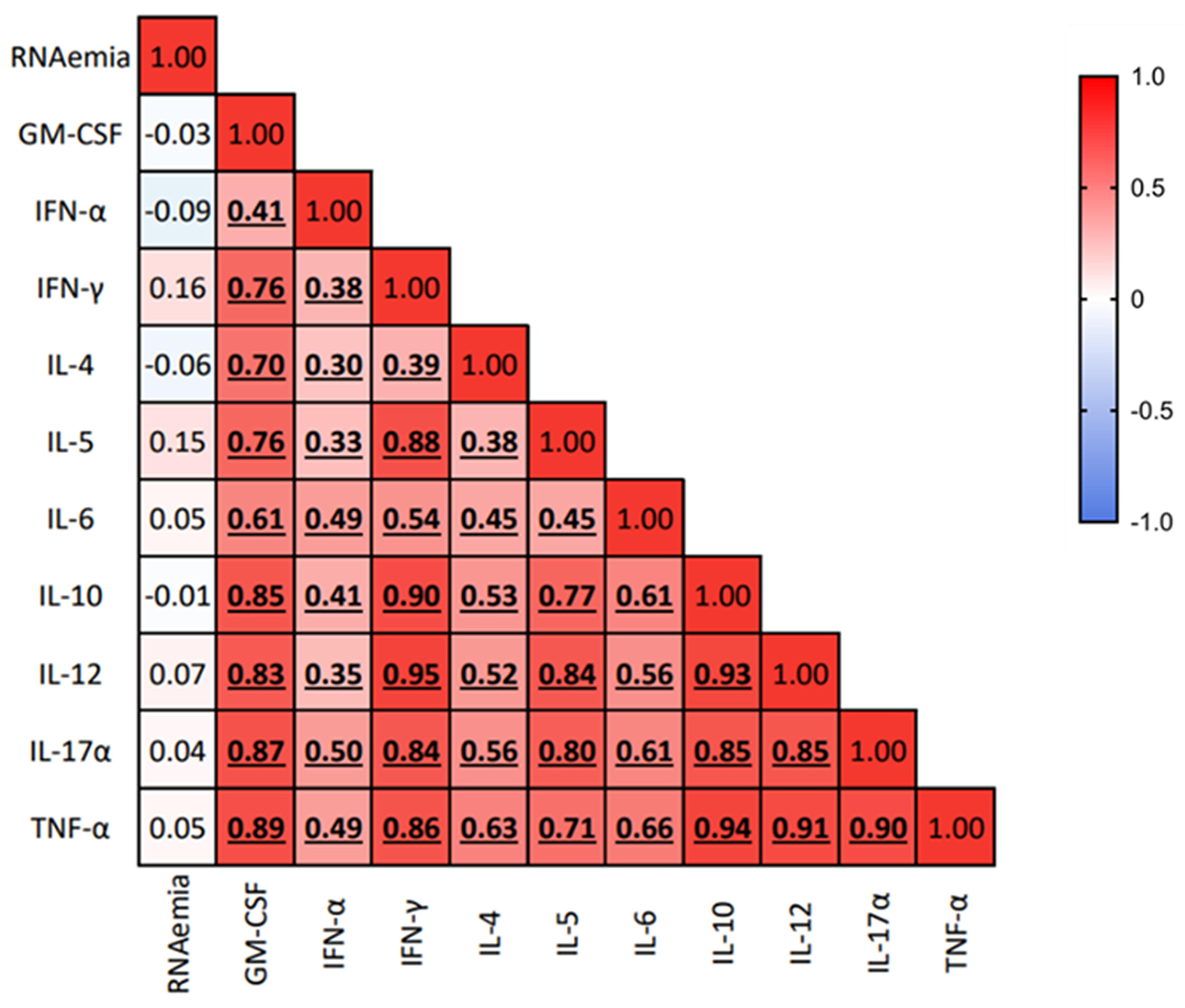
Figure 3. Association of SARS-CoV-2 RNAemia with the peripheral cytokine milieu. Heatmap showing correlations between SARS-CoV-2 RNAemia log10(copies/mL) and plasmatic chemokine and cytokine levels (pg/mL). Spearman’s correlation test was used to assess correlation between variables; the color gradient represents the correlation coefficient (r); numerical values within each cell indicate the r value. P-values <0.05 are considered statistically significant, and statistically significant correlations are highlighted in bold and underlined.
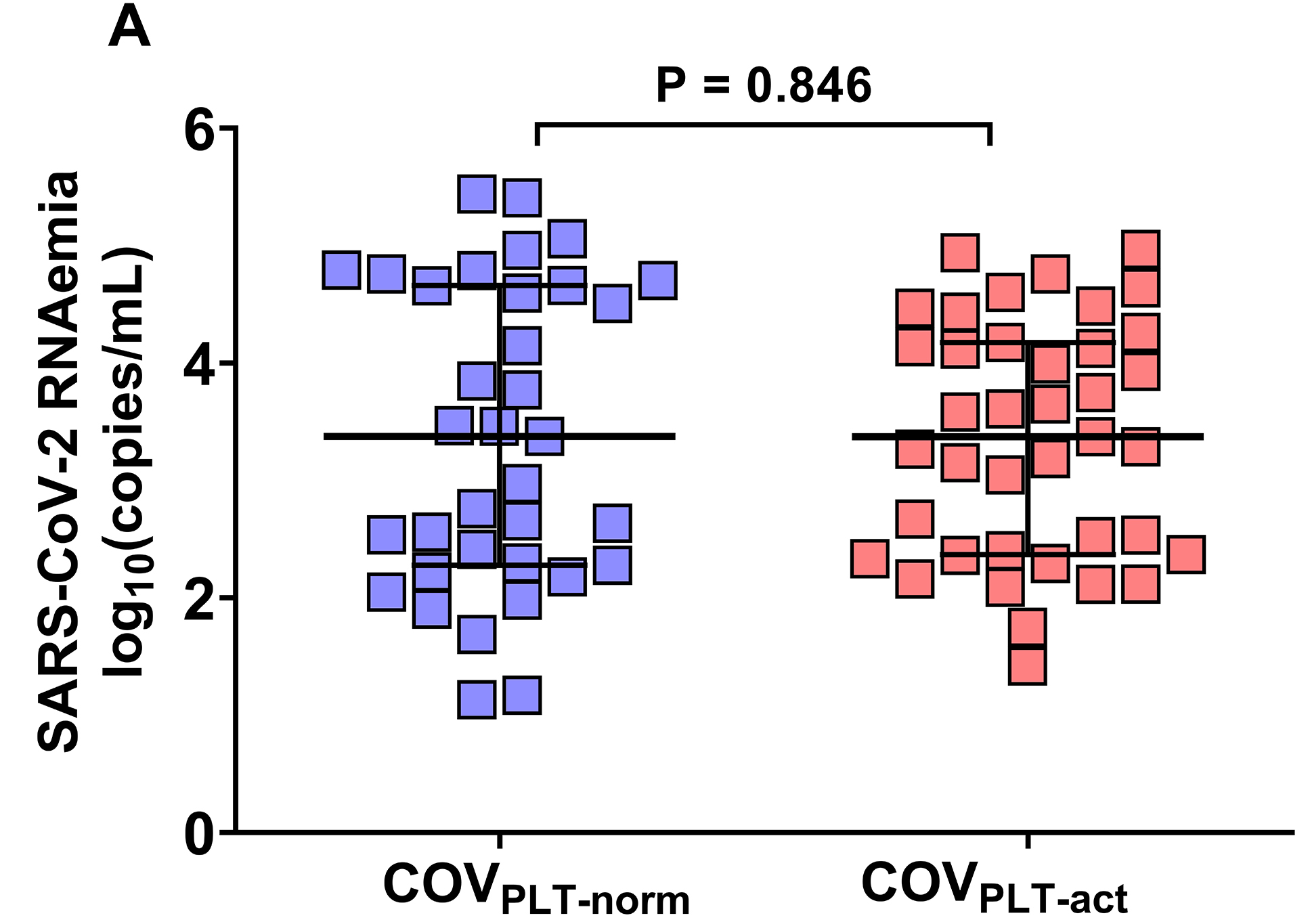
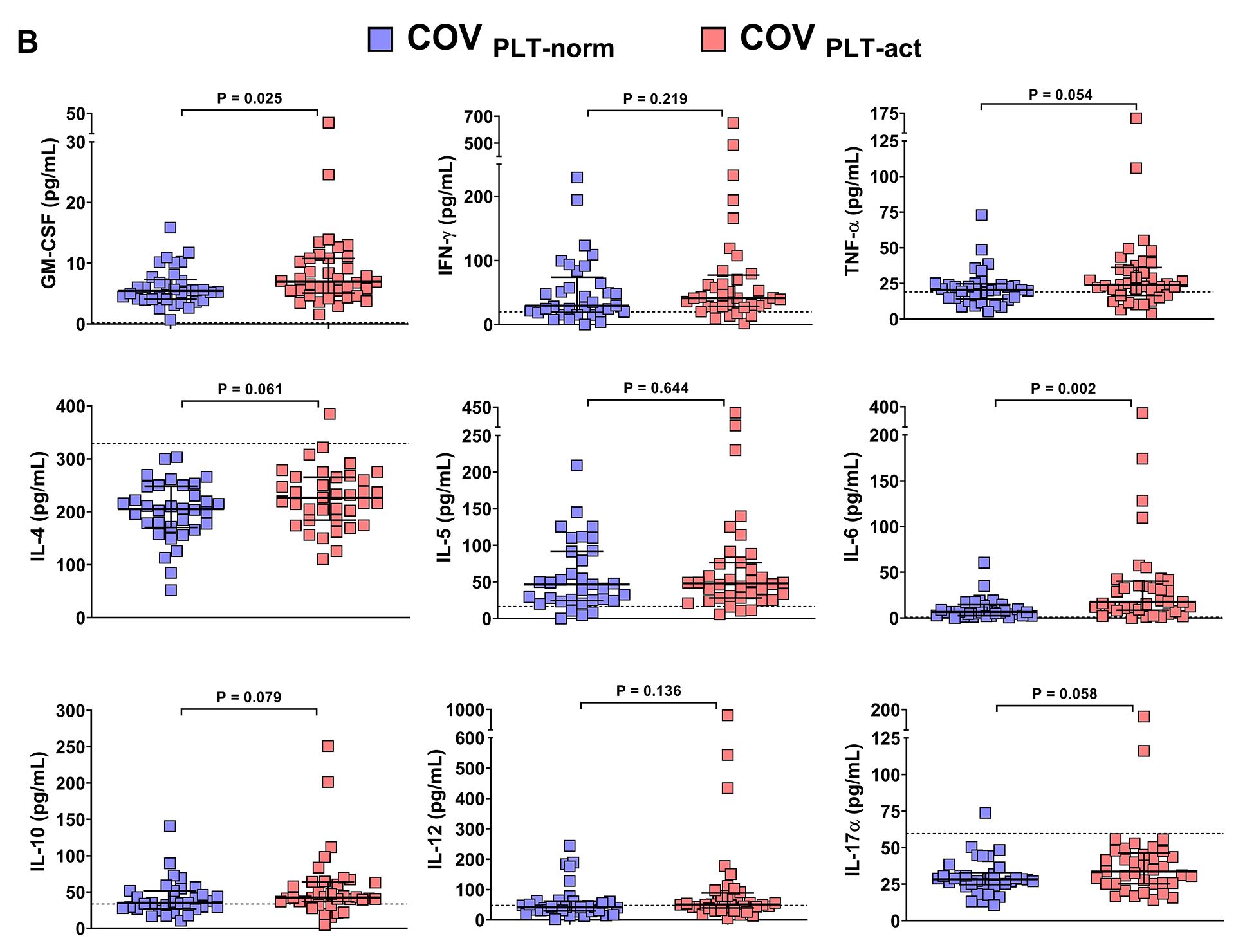
Figure 4. Association of platelet activation with SARS-CoV-2 RNAemia and the peripheral cytokine milieu. Patients with COVID-19 were categorized into two groups according to the cut-off for PMAs measured in 36 healthy subjects: COVPLT-norm (n=39) had PMA levels below the cut-off of 23.02%, whereas COVPLT-act (n= 35) had PMA levels above the cut-off. (A) Plasmatic SARS-CoV-2 RNAemia in patients with COVID-19 without circulating activated platelets (COVPLT-norm, n=35) and with circulating activated platelets (COVPLT-act, n=39) [COVPLT-norm: 3.4 median log10(copies/mL), IQR 1.4-4.2, vs COVPLT-act: 3.4 median log10(copies/mL), IQR 2.3-4.7, respectively, p=0.846]. (B) Plasmatic chemokine and cytokine levels in COVID-19 patients without circulating activated platelets (COVPLT-norm) and with circulating activated platelets (COVPLT-act). GM-CSF (n=34 and n=37), IFN-γ (n=33 and n=36), TNF-α (n=33 and n=37), IL-4 (n=34 and n=37), IL-5 (n=33 and n=36), IL-6 (n=33 and n=36), IL-10 (n=32 and n=36), IL-12 (n=33 and n=36), IL-17A (n=34 and n=37). Median values with interquartile ranges (IQR) are shown for each group of patients. Data were analyzed using the Mann–Whitney or t-test, as appropriate, and the Spearman’s correlation test. Statistical significance has been assumed for two-sided P-values <0.05.
In this study, we investigated the complex interplay between SARS-CoV-2 RNAemia and systemic inflammation in the onset of platelet dysfunction in a cohort of patients with COVID-19 during the acute phase of the disease. We and others have described the presence of circulating degranulated platelets in patients with COVID-19 [9, 21] and wondered if their presence might be a hallmark of the alterations of the haemostatic system leading to both thrombosis and bleeding in these patients. The mechanisms leading to the detection of circulating degranulated platelets during acute SARS-CoV-2 infection, however, remain obscure. According to our findings, although SARS-CoV-2 RNAemia was detectable in the blood of patients with COVID-19, it was not associated with platelet dysfunction or platelet activation. We have found that plasma levels of cytokines and chemokines were strongly associated with the investigated parameters of platelet dysfunction. Thus, our results suggest that peripheral inflammation is more strongly associated with platelet dysfunction than the levels of SARS-CoV-2 RNAemia.
The detection of SARS-CoV-2 RNAemia has been a topic of considerable interest, as it may reflect viral dissemination beyond the respiratory tract [33] and has been associated with poor clinical outcomes and immune dysfunction [14, 31, 34]. In our cohort, SARS-CoV-2 RNAemia was detected across a wide range of patients with varying disease severity (ie, both outpatients and hospitalized). However, RNAemia did not show a statistically significant correlation with platelet degranulation. Therefore, the presence of viral RNA in the bloodstream alone may not be directly linked to the platelet dysfunction observed in patients with COVID-19. However, it is plausible that SARS-CoV-2 RNAemia might affect platelet function through other pathways, such as endothelial damage or immune activation, which were not investigated in this study.
In contrast, we found that pro-inflammatory cytokines and chemokines were significantly elevated in patients with circulating degranulated platelets, with strong correlations between the levels of IL-6 and GM-CSF and parameters of platelet dysfunction. These findings point towards a crucial role of peripheral inflammation in platelet dysfunction of COVID-19, with cytokines possibly acting in synergy with mediators like ADP or thromboxane A2 [35–37] to exacerbate the prothrombotic state. Although platelets do not directly produce the cytokine IL-6, which was measured in our study, they can still indirectly contribute to its systemic levels. Notably, activated platelets can release IL-1β, a potent pro-inflammatory cytokine capable of stimulating endothelial cells, monocytes [38], and other immune cells to produce secondary cytokines, including IL-6. Therefore, while platelets are not a direct source of IL-6, they may contribute to its upregulation via the release of upstream mediators.
The clinical implications of these findings are significant. Platelet dysfunction has been associated with thrombotic [6, 39–43] and bleeding complications [5] in severe COVID-19. Our findings suggest the potential benefit of targeting inflammation to prevent or mitigate platelet-related complications in COVID-19.
Our study has limitations that should be acknowledged. Firstly, the sample size, and particularly that of the COVδ-low subgroup, was relatively small. Additionally, we did not directly quantify SARS-CoV-2 RNA within platelets, thus our study does not contribute to the unresolved question of whether platelets are directly infected by the virus. It is also important to note that our study excluded patients on antiplatelet treatment, so our findings cannot be extended to this population and do not clarify whether pre-existing antiplatelet therapy alleviates or exacerbates platelet dysfunction in COVID-19 patients. Finally, although we focused on the presence of circulating degranulated and activated platelets, we acknowledge that many other contributors (eg, endothelial dysfunction, von Willebrand factor activity, thrombin generation) play a role in the onset of thrombotic and bleeding complications in patients with COVID-19.
In conclusion, our study provides new insights into the pathophysiology of platelet dysfunction in COVID-19, emphasizing the pivotal role of inflammation, rather than RNAemia, in driving these changes. Our findings suggest that early therapeutic strategies aimed at controlling inflammation may be crucial for the prevention of the thrombotic and bleeding complications seen in severe COVID-19. Further research is needed to deepen our understanding of the mechanisms linking inflammation to platelet dysfunction in COVID-19 as well as in other viral and bacterial infections, with the final aim of developing targeted interventions that could improve patient outcomes.
MS and RR contributed to the design of the study, performed laboratory analysis, analyzed the data, and wrote the manuscript; CG, AF, AS, EB, and EB performed laboratory analysis, and analyzed the data; BC and CT contributed to literature search, manuscript writing, and figure design; MC and GP designed the study, coordinated the group, contributed to the interpretation of the data analysis, and wrote and revised the manuscript. All authors read and approved the final version of the manuscript.
The authors are thankful to all the participants of the study and to the medical/nursing staff of hospital San Paolo, ASST Santi Paolo e Carlo, whose cooperation was essential for the collection of samples for this study.
This work was partially supported by a grant from the Dipartimento DISS, Linea 2 Piano di sostegno della ricerca – Università degli studi di Milano. This work was supported by grant from the EuCARE Project under Grant Agreement No 101046016, which is part of the European Union´s Horizon Europe Research and Innovation Programme.
The authors declare no competing interests.
1. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS, China Medical Treatment Expert Group for C. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708-20. Epub 20200228. doi: 10.1056/NEJMoa2002032. PubMed PMID: 32109013; PMCID: PMC7092819.
2. Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239-42. doi: 10.1001/jama.2020.2648. PubMed PMID: 32091533.
3. Thomas MR, Scully M. Clinical features of thrombosis and bleeding in COVID-19. Blood. 2022;140(3):184-95. doi: 10.1182/blood.2021012247. PubMed PMID: 35452509; PMCID: PMC9040438.
4. Hanley B, Naresh KN, Roufosse C, Nicholson AG, Weir J, Cooke GS, Thursz M, Manousou P, Corbett R, Goldin R, Al-Sarraj S, Abdolrasouli A, Swann OC, Baillon L, Penn R, Barclay WS, Viola P, Osborn M. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. Lancet Microbe. 2020;1(6):e245-e53. Epub 20200820. doi: 10.1016/S2666-5247(20)30115-4. PubMed PMID: 32844161; PMCID: PMC7440861.
5. Musoke N, Lo KB, Albano J, Peterson E, Bhargav R, Gul F, DeJoy R, 3rd, Salacup G, Pelayo J, Tipparaju P, Azmaiparashvili Z, Patarroyo-Aponte G, Rangaswami J. Anticoagulation and bleeding risk in patients with COVID-19. Thromb Res. 2020;196:227-30. Epub 20200824. doi: 10.1016/j.thromres.2020.08.035. PubMed PMID: 32916565; PMCID: PMC7444469.
6. Barrett TJ, Lee AH, Xia Y, Lin LH, Black M, Cotzia P, Hochman J, Berger JS. Platelet and Vascular Biomarkers Associate With Thrombosis and Death in Coronavirus Disease. Circ Res. 2020;127(7):945-7. Epub 20200806. doi: 10.1161/CIRCRESAHA.120.317803. PubMed PMID: 32757722; PMCID: PMC7478197.
7. Hottz ED, Azevedo-Quintanilha IG, Palhinha L, Teixeira L, Barreto EA, Pao CRR, Righy C, Franco S, Souza TML, Kurtz P, Bozza FA, Bozza PT. Platelet activation and platelet-monocyte aggregate formation trigger tissue factor expression in patients with severe COVID-19. Blood. 2020;136(11):1330-41. doi: 10.1182/blood.2020007252. PubMed PMID: 32678428; PMCID: PMC7483437.
8. Manne BK, Denorme F, Middleton EA, Portier I, Rowley JW, Stubben C, Petrey AC, Tolley ND, Guo L, Cody M, Weyrich AS, Yost CC, Rondina MT, Campbell RA. Platelet gene expression and function in patients with COVID-19. Blood. 2020;136(11):1317-29. doi: 10.1182/blood.2020007214. PubMed PMID: 32573711; PMCID: PMC7483430.
9. Scavone M, Ghali C, Calogiuri M, Sala M, Bossi E, Mencarini T, Bozzi S, Clerici B, Birocchi S, Fioretti A, Bono V, Maugeri N, Marchetti G, Cattaneo M, Podda GM. Impairment of platelet function in both mild and severe COVID-19 patients. Br J Haematol. 2023;203(4):656-67. Epub 20230824. doi: 10.1111/bjh.19062. PubMed PMID: 37615207.
10. Lindner D, Fitzek A, Brauninger H, Aleshcheva G, Edler C, Meissner K, Scherschel K, Kirchhof P, Escher F, Schultheiss HP, Blankenberg S, Puschel K, Westermann D. Association of Cardiac Infection With SARS-CoV-2 in Confirmed COVID-19 Autopsy Cases. JAMA Cardiol. 2020;5(11):1281-5. doi: 10.1001/jamacardio.2020.3551. PubMed PMID: 32730555; PMCID: PMC7385672.
11. Qian Q, Fan L, Liu W, Li J, Yue J, Wang M, Ke X, Yin Y, Chen Q, Jiang C. Direct Evidence of Active SARS-CoV-2 Replication in the Intestine. Clin Infect Dis. 2021;73(3):361-6. doi: 10.1093/cid/ciaa925. PubMed PMID: 32638022; PMCID: PMC7454471.
12. Xiang Q, Feng Z, Diao B, Tu C, Qiao Q, Yang H, Zhang Y, Wang G, Wang H, Wang C, Liu L, Wang C, Liu L, Chen R, Wu Y, Chen Y. SARS-CoV-2 Induces Lymphocytopenia by Promoting Inflammation and Decimates Secondary Lymphoid Organs. Front Immunol. 2021;12:661052. Epub 20210428. doi: 10.3389/fimmu.2021.661052. PubMed PMID: 33995382; PMCID: PMC8113960.
13. Puelles VG, Lutgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L, Chilla S, Heinemann A, Wanner N, Liu S, Braun F, Lu S, Pfefferle S, Schroder AS, Edler C, Gross O, Glatzel M, Wichmann D, Wiech T, Kluge S, Pueschel K, Aepfelbacher M, Huber TB. Multiorgan and Renal Tropism of SARS-CoV-2. N Engl J Med. 2020;383(6):590-2. Epub 20200513. doi: 10.1056/NEJMc2011400. PubMed PMID: 32402155; PMCID: PMC7240771.
14. Rovito R, Bono V, Augello M, Tincati C, Mainoldi F, Beaudoin-Bussieres G, Tauzin A, Bianchi S, Hadla M, Yellenki V, d’Arminio Monforte A, Casola S, Borghi E, Finzi A, Marchetti G. Association between SARS-CoV-2 RNAemia and dysregulated immune response in acutely ill hospitalized COVID-19 patients. Sci Rep. 2022;12(1):19658. Epub 20221116. doi: 10.1038/s41598-022-23923-1. PubMed PMID: 36385627; PMCID: PMC9667450.
15. Fajnzylber J, Regan J, Coxen K, Corry H, Wong C, Rosenthal A, Worrall D, Giguel F, Piechocka-Trocha A, Atyeo C, Fischinger S, Chan A, Flaherty KT, Hall K, Dougan M, Ryan ET, Gillespie E, Chishti R, Li Y, Jilg N, Hanidziar D, Baron RM, Baden L, Tsibris AM, Armstrong KA, Kuritzkes DR, Alter G, Walker BD, Yu X, Li JZ, Massachusetts Consortium for Pathogen R. SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat Commun. 2020;11(1):5493. Epub 20201030. doi: 10.1038/s41467-020-19057-5. PubMed PMID: 33127906; PMCID: PMC7603483.
16. Brunet-Ratnasingham E, Anand SP, Gantner P, Dyachenko A, Moquin-Beaudry G, Brassard N, Beaudoin-Bussieres G, Pagliuzza A, Gasser R, Benlarbi M, Point F, Prevost J, Laumaea A, Niessl J, Nayrac M, Sannier G, Orban C, Messier-Peet M, Butler-Laporte G, Morrison DR, Zhou S, Nakanishi T, Boutin M, Descoteaux-Dinelle J, Gendron-Lepage G, Goyette G, Bourassa C, Medjahed H, Laurent L, Rebillard RM, Richard J, Dube M, Fromentin R, Arbour N, Prat A, Larochelle C, Durand M, Richards JB, Chasse M, Tetreault M, Chomont N, Finzi A, Kaufmann DE. Integrated immunovirological profiling validates plasma SARS-CoV-2 RNA as an early predictor of COVID-19 mortality. Sci Adv. 2021;7(48):eabj5629. Epub 20211126. doi: 10.1126/sciadv.abj5629. PubMed PMID: 34826237; PMCID: PMC8626074.
17. Chen X, Zhao B, Qu Y, Chen Y, Xiong J, Feng Y, Men D, Huang Q, Liu Y, Yang B, Ding J, Li F. Detectable Serum Severe Acute Respiratory Syndrome Coronavirus 2 Viral Load (RNAemia) Is Closely Correlated With Drastically Elevated Interleukin 6 Level in Critically Ill Patients With Coronavirus Disease 2019. Clin Infect Dis. 2020;71(8):1937-42. doi: 10.1093/cid/ciaa449. PubMed PMID: 32301997; PMCID: PMC7184354.
18. Li Y, Schneider AM, Mehta A, Sade-Feldman M, Kays KR, Gentili M, Charland NC, Gonye ALK, Gushterova I, Khanna HK, LaSalle TJ, Lavin-Parsons KM, Lilly BM, Lodenstein CL, Manakongtreecheep K, Margolin JD, McKaig BN, Parry BA, Rojas-Lopez M, Russo BC, Sharma N, Tantivit J, Thomas MF, Regan J, Flynn JP, Villani AC, Hacohen N, Goldberg MB, Filbin MR, Li JZ. SARS-CoV-2 Viremia is Associated with Distinct Proteomic Pathways and Predicts COVID-19 Outcomes. medRxiv. 2021. Epub 20210226. doi: 10.1101/2021.02.24.21252357. PubMed PMID: 33655257; PMCID: PMC7924277.
19. Rodriguez-Serrano DA, Roy-Vallejo E, Zurita Cruz ND, Martin Ramirez A, Rodriguez-Garcia SC, Arevalillo-Fernandez N, Galvan-Roman JM, Fontan Garcia-Rodrigo L, Vega-Piris L, Chicot Llano M, Arribas Mendez D, Gonzalez de Marcos B, Hernando Santos J, Sanchez Azofra A, Avalos Perez-Urria E, Rodriguez-Cortes P, Esparcia L, Marcos-Jimenez A, Sanchez-Alonso S, Llorente I, Soriano J, Suarez Fernandez C, Garcia-Vicuna R, Ancochea J, Sanz J, Munoz-Calleja C, de la Camara R, Canabal Berlanga A, Gonzalez-Alvaro I, Cardenoso L, Group R-C. Detection of SARS-CoV-2 RNA in serum is associated with increased mortality risk in hospitalized COVID-19 patients. Sci Rep. 2021;11(1):13134. Epub 20210623. doi: 10.1038/s41598-021-92497-1. PubMed PMID: 34162948; PMCID: PMC8222315.
20. Perumal R, Shunmugam L, Naidoo K, Wilkins D, Garzino-Demo A, Brechot C, Vahlne A, Nikolich J. Biological mechanisms underpinning the development of long COVID. iScience. 2023;26(6):106935. Epub 20230518. doi: 10.1016/j.isci.2023.106935. PubMed PMID: 37265584; PMCID: PMC10193768.
21. Zaid Y, Puhm F, Allaeys I, Naya A, Oudghiri M, Khalki L, Limami Y, Zaid N, Sadki K, Ben El Haj R, Mahir W, Belayachi L, Belefquih B, Benouda A, Cheikh A, Langlois MA, Cherrah Y, Flamand L, Guessous F, Boilard E. Platelets Can Associate with SARS-Cov-2 RNA and Are Hyperactivated in COVID-19. Circ Res. 2020;127(11):1404-18. Epub 20200917. doi: 10.1161/CIRCRESAHA.120.317703. PubMed PMID: 32938299; PMCID: PMC7641188.
22. Zhu A, Real F, Capron C, Rosenberg AR, Silvin A, Dunsmore G, Zhu J, Cottoignies-Callamarte A, Masse JM, Moine P, Bessis S, Godement M, Geri G, Chiche JD, Valdebenito S, Belouzard S, Dubuisson J, Lorin de la Grandmaison G, Chevret S, Ginhoux F, Eugenin EA, Annane D, Borde EC, Bomsel M. Infection of lung megakaryocytes and platelets by SARS-CoV-2 anticipate fatal COVID-19. Cell Mol Life Sci. 2022;79(7):365. Epub 20220616. doi: 10.1007/s00018-022-04318-x. PubMed PMID: 35708858; PMCID: PMC9201269.
23. Campbell RA, Schwertz H, Hottz ED, Rowley JW, Manne BK, Washington AV, Hunter-Mellado R, Tolley ND, Christensen M, Eustes AS, Montenont E, Bhatlekar S, Ventrone CH, Kirkpatrick BD, Pierce KK, Whitehead SS, Diehl SA, Bray PF, Zimmerman GA, Kosaka Y, Bozza PT, Bozza FA, Weyrich AS, Rondina MT. Human megakaryocytes possess intrinsic antiviral immunity through regulated induction of IFITM3. Blood. 2019;133(19):2013-26. Epub 20190205. doi: 10.1182/blood-2018-09-873984. PubMed PMID: 30723081; PMCID: PMC6509546.
24. Real F, Capron C, Sennepin A, Arrigucci R, Zhu A, Sannier G, Zheng J, Xu L, Masse JM, Greffe S, Cazabat M, Donoso M, Delobel P, Izopet J, Eugenin E, Gennaro ML, Rouveix E, Cramer Borde E, Bomsel M. Platelets from HIV-infected individuals on antiretroviral drug therapy with poor CD4(+) T cell recovery can harbor replication-competent HIV despite viral suppression. Sci Transl Med. 2020;12(535). doi: 10.1126/scitranslmed.aat6263. PubMed PMID: 32188724.
25. Vogt MB, Lahon A, Arya RP, Spencer Clinton JL, Rico-Hesse R. Dengue viruses infect human megakaryocytes, with probable clinical consequences. PLoS Negl Trop Dis. 2019;13(11):e0007837. Epub 20191125. doi: 10.1371/journal.pntd.0007837. PubMed PMID: 31765380; PMCID: PMC6901235.
26. Zhang S, Liu Y, Wang X, Yang L, Li H, Wang Y, Liu M, Zhao X, Xie Y, Yang Y, Zhang S, Fan Z, Dong J, Yuan Z, Ding Z, Zhang Y, Hu L. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J Hematol Oncol. 2020;13(1):120. Epub 20200904. doi: 10.1186/s13045-020-00954-7. PubMed PMID: 32887634; PMCID: PMC7471641.
27. Shen S, Zhang J, Fang Y, Lu S, Wu J, Zheng X, Deng F. SARS-CoV-2 interacts with platelets and megakaryocytes via ACE2-independent mechanism. J Hematol Oncol. 2021;14(1):72. Epub 20210429. doi: 10.1186/s13045-021-01082-6. PubMed PMID: 33926500; PMCID: PMC8082485.
28. Carnevale R, Cammisotto V, Bartimoccia S, Nocella C, Castellani V, Bufano M, Loffredo L, Sciarretta S, Frati G, Coluccia A, Silvestri R, Ceccarelli G, Oliva A, Venditti M, Pugliese F, Maria Mastroianni C, Turriziani O, Leopizzi M, D’Amati G, Pignatelli P, Violi F. Toll-Like Receptor 4-Dependent Platelet-Related Thrombosis in SARS-CoV-2 Infection. Circ Res. 2023;132(3):290-305. Epub 20230113. doi: 10.1161/CIRCRESAHA.122.321541. PubMed PMID: 36636919.
29. Singh A, Bisht P, Bhattacharya S, Guchhait P. Role of Platelet Cytokines in Dengue Virus Infection. Front Cell Infect Microbiol. 2020;10:561366. Epub 20200930. doi: 10.3389/fcimb.2020.561366. PubMed PMID: 33102253; PMCID: PMC7554584.
30. Lumadue JA, Lanzkron SM, Kennedy SD, Kuhl DT, Kickler TS. Cytokine induction of platelet activation. Am J Clin Pathol. 1996;106(6):795-8. doi: 10.1093/ajcp/106.6.795. PubMed PMID: 8980357.
31. Rovito R, Augello M, Ben-Haim A, Bono V, d’Arminio Monforte A, Marchetti G. Hallmarks of Severe COVID-19 Pathogenesis: A Pas de Deux Between Viral and Host Factors. Front Immunol. 2022;13:912336. Epub 20220610. doi: 10.3389/fimmu.2022.912336. PubMed PMID: 35757770; PMCID: PMC9231592.
32. Michelson AD. Methods for the measurement of platelet function. Am J Cardiol. 2009;103 (3 Suppl):20A-6A. doi: 10.1016/j.amjcard.2008.11.019. PubMed PMID: 19166709.
33. Hogan CA, Stevens BA, Sahoo MK, Huang C, Garamani N, Gombar S, Yamamoto F, Murugesan K, Kurzer J, Zehnder J, Pinsky BA. High Frequency of SARS-CoV-2 RNAemia and Association With Severe Disease. Clin Infect Dis. 2021;72(9):e291-e5. doi: 10.1093/cid/ciaa1054. PubMed PMID: 32965474; PMCID: PMC7543277.
34.Rovito R, Bono V, Coianiz N, Cazzetta V, Franzese S, Mikulak J, Di Vito C, Bai F, Beaudoin-Bussières G, Tauzin A, Augello M, Tincati C, Santoro A, Borghi E, Marozin S, Finzi A, Della Bella S, Mavilio D, Marchetti G; EuCare Study Group. Multi-layered deep immune profiling, SARS-CoV-2 RNAemia and inflammation in unvaccinated COVID-19 individuals with persistent symptoms. Commun Med (Lond). 2025 May 5;5(1):155. doi: 10.1038/s43856-025-00832-8. PMID: 40325175; PMCID: PMC12052991.
35. Maione F, Cicala C, Liverani E, Mascolo N, Perretti M, D’Acquisto F. IL-17A increases ADP-induced platelet aggregation. Biochem Biophys Res Commun. 2011;408(4):658-62. Epub 20110422. doi: 10.1016/j.bbrc.2011.04.080. PubMed PMID: 21530487; PMCID: PMC3182527.
36. Canzano P, Brambilla M, Porro B, Cosentino N, Tortorici E, Vicini S, Poggio P, Cascella A, Pengo MF, Veglia F, Fiorelli S, Bonomi A, Cavalca V, Trabattoni D, Andreini D, Omodeo Sale E, Parati G, Tremoli E, Camera M. Platelet and Endothelial Activation as Potential Mechanisms Behind the Thrombotic Complications of COVID-19 Patients. JACC Basic Transl Sci. 2021;6(3):202-18. Epub 20210224. doi: 10.1016/j.jacbts.2020.12.009. PubMed PMID: 33649738; PMCID: PMC7904280.
37. Marino M, Scuderi F, Ponte E, Maiuri MT, De Cristofaro R, Provenzano C, Rose-John S, Cittadini A, Bartoccioni E. Novel path to IL-6 trans-signaling through thrombin-induced soluble IL-6 receptor release by platelets. J Biol Regul Homeost Agents. 2013;27(3):841-52. PubMed PMID: 24152848.
38. Tosato G, Jones KD. Interleukin-1 induces interleukin-6 production in peripheral blood monocytes. Blood. 1990;75(6):1305-10. PubMed PMID: 2310829.
39. Klok FA, Kruip M, van der Meer NJM, Arbous MS, Gommers D, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, Endeman H. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thromb Res. 2020;191:148-50. Epub 20200430. doi: 10.1016/j.thromres.2020.04.041. PubMed PMID: 32381264; PMCID: PMC7192101.
40. Liu Y, Sun W, Guo Y, Chen L, Zhang L, Zhao S, Long D, Yu L. Association between platelet parameters and mortality in coronavirus disease 2019: Retrospective cohort study. Platelets. 2020;31(4):490-6. Epub 20200416. doi: 10.1080/09537104.2020.1754383. PubMed PMID: 32297540; PMCID: PMC7171387.
41. Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, Kucher N, Studt JD, Sacco C, Bertuzzi A, Sandri MT, Barco S, Humanitas C-TF. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9-14. Epub 20200423. doi: 10.1016/j.thromres.2020.04.024. PubMed PMID: 32353746; PMCID: PMC7177070.
42. Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, Merdji H, Clere-Jehl R, Schenck M, Fagot Gandet F, Fafi-Kremer S, Castelain V, Schneider F, Grunebaum L, Angles-Cano E, Sattler L, Mertes PM, Meziani F, Group CT. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089-98. Epub 20200504. doi: 10.1007/s00134-020-06062-x. PubMed PMID: 32367170; PMCID: PMC7197634.
43. Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18(6):1421-4. Epub 20200506. doi: 10.1111/jth.14830. PubMed PMID: 32271988; PMCID: PMC7262324.
Submitted April 16, 2025 | Accepted May 27, 2025 | Published July 9, 2025
Copyright © 2025 The Authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License.