Anna-Maria Gierke1, Martin Hessling1
1Institute of Medical Engineering and Mechatronics, Ulm University of Applied Sciences, Ulm, Germany
Anna-Maria Gierke
anna-maria.gierke@thu.de
Gierke AM, Hessling M. Sensitivity Analysis of C. auris, S. cerevisiae, and C. cladosporioides by Irradiation with Far-UVC, UVC, and UVB. Pathogens and Immunity. 2024;9(2):135–151. doi: 10.20411/pai.v9i2.723
10.20411/pai.v9i2.723
Background: The World Health Organization has published a list of pathogenic fungi with prioritizing groups and calls for research and development of antifungal measures, with Candida auris belonging to the group with high priority.
Methods: The photosensitivity towards short wavelength ultraviolet irradiation (Far-UVC, UVC, and UVB) was investigated and compared to other yeasts (Saccharomyces cerevisiae) and a mold (Cladosporium cladosporioides). The observed 1-log reduction doses were compared to literature values of other representatives of the genus Candida, but also with S. cerevisiae, Aspergillus niger, and A. fumigatus.
Results: For the determined 1-log reduction doses, an increase with higher wavelengths was observed. A 1-log reduction dose of 4.3 mJ/cm2 was determined for C. auris when irradiated at 222 nm, a dose of 6.1 mJ/cm2 at 254 nm and a 1-log reduction dose of 51.3 mJ/cm2 was required when irradiated with UVB.
Conclusions: It was observed that S. cerevisiae is a possible surrogate for C. auris for irradiation with Far-UVC and UVB due to close 1-log reduction doses. No surrogate suitability was verified for C. cladosporioides in relation to A. niger and A. fumigatus for irradiation with a wavelength of 254 nm and for A. niger at 222 nm.
222 nm; 254 nm; 302 nm; disinfection; photoinactivation; surrogate
In 2022, the World Health Organization (WHO) published a document listing important fungal pathogens in groups of different importance. Candida auris was classified with C. albicans, Aspergillus fumigatus and Cryptococcus neoformans as the critical priority group [1]. Due to the increasing development of antifungal drug resistance, treatment of infection has become more difficult [2–5]. Candida spp. are known to cause invasive fungal infections (IFIs) [2, 6, 7]. In addition to the very wide-spread representative Candida albicans, the number of infected persons and deaths worldwide due to C. auris have been increasing over the last few years. As was first described in Japan, C. auris has been discovered in hospitals and healthcare facilities in more countries [2, 8–10]. It is a lethal threat, especially to immunocompromised patients [11, 12].
In addition to antimycotics, there are also studies on chemical disinfectants that are intended to reduce the spread of nosocomial infections [13–15]. Another possibility for the reduction of C. auris and other pathogenic fungi is irradiation at different wavelengths. For the selected spectral ranges, damage to humans by application of this radiation to different body areas is described by Ramasamy et al [16]. Due to short wavelength UV irradiation, cyclobutane pyrimidine dimers (CPD) and 6-4 photoproducts in DNA (deoxyribonucleic acid) are formed, which can result in mutagenic effects or even cell death [16–21].
In the case of UVC, however, a further distinction should be made on the basis of recent studies on the effect of the so-called Far-UVC (200 - 230 nm) [22, 23]. In contrast to an irradiation at 254 nm, which is hazardous to humans, an application of Far-UVC radiation on human skin and eyes appears less harmful [16, 17, 24, 25] and might even allow radiation disinfection in the presence of humans.
In this study, the 1-log reduction doses for C. auris are determined and compared with the D90 values of other representatives of the genus Candida, another yeast (S. cerevisiae) and molds with pigmented spores (C. cladosporioides, A. niger and A. fumigatus). The experiments are carried out in transparent liquid suspensions to obtain information about photoinactivation based only on fungal properties. Further 1-log reduction doses are taken from other studies with similar experimental setups. The aim is to make statements about possible surrogates for irradiation experiments for C. auris and C. albicans or also about similarities and differences between the examined fungi. Furthermore, it should be investigated whether there is a possible connection between pathogenicity and radiation sensitivity.
When irradiating the pigmented spores of the mold, 1-log reduction doses for C. cladosporioides that are many times higher are expected and compared to A. niger, whose genus is also classified as potentially pathogenic [1, 26, 27] and A. fumigatus, which is listed by WHO in the critical priority group [1]. This is intended to consider whether C. cladosporioides could be a possible surrogate for A. niger or A. fumigatus. C. cladosporioides is chosen for experiments due to its very wide distribution and pigmented spores protecting against radiation damage.
The following fungal strains were investigated in the irradiation experiments: C. auris (DSM 21092, clade II, ATCC MYA-5001, strain designation B11220), S. cerevisiae (DSM 70449) and C. cladosporioides (DSM 19653). Since the present laboratory has a biosafety level of 1 and work with pathogenic representatives of yeasts and molds is only permitted in laboratories with higher safety standards, these fungi were selected.
For the 2 yeasts C. auris and S. cerevisiae, modified YEPG (Yeast Extract Peptone Glucose medium - 200 ml glucose (250 g/l), 20 g peptone from casein, 10 g yeast extract per 1,000 ml; pH value of 6.5) was used for the liquid cultures and potato dextrose agar (M129 – 20 g dextrose, 4 g potato extract, [15 g agar] per 1,000 ml; pH 5.6) for the agar plates. For the mold C. cladosporioides, M90 (30 g malt extract, 3 g soya peptone, [15 g agar] per 1,000 ml; pH 5.6) was chosen for agar plates and liquid cultures. Both yeasts were cultured at 30 °C until mid-exponential phase resulting in a population density of around 3 x 109 CFU/ml (colony forming units per ml). After reaching the growth phase, 3 ml of the culture was removed and centrifuged at 7,000 rpm for 5 minutes. The resulting supernatant was discarded and the pellet resuspended in phosphate buffered saline (PBS). The washing procedure was repeated twice. The sample was then diluted to a population density of 5 x 106 to 1 x 107 colony forming units (CFU) per mL. The sample transmission for the irradiation wavelength was measured in a 10 mm quartz cuvette using a spectrophotometer (SPECORD 250 PLUS double beam spectrophotometer, Analytik Jena GmbH+Co. KG) to ensure a transmission of 70% to 80% for a sample thickness of 3 mm, the sample height during irradiation. In the case of the mold, only the spores were investigated. For this purpose, spores were previously spread on an agar plate and allowed to grow at 24 °C for 4 days. A solution of 0.9% NaCl and 0.01% TWEEN 80 was then added to the overgrown plate to brush over the mycelium with a sterile inoculation loop. During this process, the spores detached and could be transferred to a sterile vessel via a funnel covered with sterile gauze [28]. The filtrate containing the spores (1 x 1010 CFU/ml) was then centrifuged at 9,000 rpm for 10 minutes and washed twice with the same solution to dissolve the spores. For the inactivation experiment, this solution was diluted until approximately 4 x 106 spores were present in 1 mL of 0.9% NaCl solution without TWEEN 80.
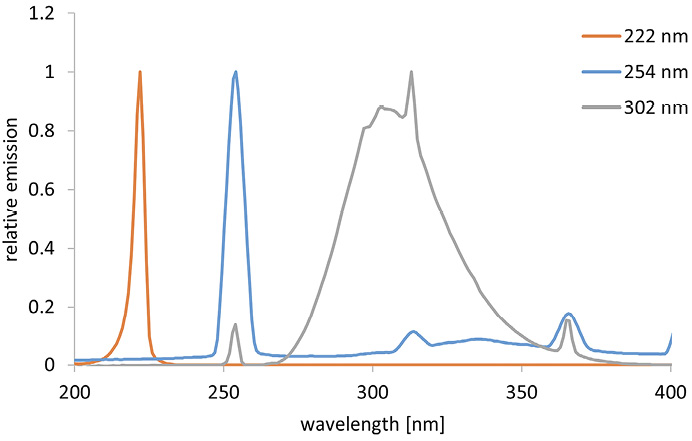
The inactivation experiments were performed with different radiation sources and different wavelengths. A krypton chloride excimer lamp (Ushio Care 222 Modell B1, Ushio Europe B.V.) with an irradiation intensity of 0.25 mW/cm2 was employed for irradiation at 222 nm and a low pressure mercury vapor lamp (TUV 15W/G15T8, Philips) with an intensity of 0.23 mW/cm2 was applied for irradiation at 254 nm. For irradiation in the UVB range (302 nm), an irradiation unit (UVP 3UV Lamp, Analytik Jena GmbH+Co. KG) with an irradiation intensity of 7.4 mW/cm2 was selected. The corresponding spectra can be found in Figure 1. The radiation source was placed over a Petri dish containing 3 mL of the sample. The control sample was placed next to the irradiated sample in the setup, covered with aluminum foil to prevent irradiation. The irradiance was measured with a photometric detector (X1-UV-3727 with X1 measuring device, Gigahertz-Optik GmbH) at the respective wavelengths (222 and 254 nm), and the UVB irradiance was determined with a spectroradiometer (CAS 140D with integrating sphere of Instrument Systems) prior to the experiments. To prevent possible photoreactivation, all samples (control and irradiation samples) and spread plates were covered with aluminum foil except during irradiation.
All experiments were performed at room temperature. After fixed periods of time, 100 µL samples of the fungal suspension were taken and the irradiation was then continued. Three samples were taken at each of the fixed times and 3 independent experimental runs were performed.
After an incubation period of 48 hours, the grown yeast colonies and after 96 hours, the germinated spores of the mold could be counted and the colony forming unit per mL were determined. The resulting logarithmic reduction values were calculated in relation to the initial value and could be graphically displayed with respective linear fit curves. Furthermore, the experiments were repeated at least 3 times with multiple dilution levels plated out and evaluated for each measurement point. The inactivation rate constants (k values) were determined from 1-log reduction doses and the reduction according to the calculation method of Lemons et al [29].

Figure 1. Relative emission spectra of the different radiation sources.
For comparison, 1-log reduction doses of various members of the genus Candida, S. cerevisiae and also of C. cladosporioides, A. niger, and A. fumigatus were selected from other published studies. As selection criterion, an open setup (Petri dishes as vessels) under aerobic condition was taken. Furthermore, the irradiation medium should have been PBS, water, or similar transparent solutions. The 1-log reduction doses were taken directly from text or tables in the literature or read from the graphs. The collected D90 values were then presented as a boxplot using Origin 2021b (Originlab Corporation).
The graphical representation of the irradiation results including the log reduction against the irradiation dose (mJ/cm2) and the ensuing linear fit can be found in Figure 2. The determined 1-log reduction doses of each wavelength and microorganism are given in Table 1.
A. 222 nm (Far-UVC)
B. 254 nm (UVC)
C. 302 nm (UVB)
Figure 2. Overview of the inactivation curves for C. auris, S. cerevisiae and C. cladosporioides.
The irradiation was carried out with different wavelengths — (A) Far-UVC, (B) UVC, and (C) UVB.
Lower 1-log reduction doses are observed for C. auris at 222 nm and 254 nm with 4.3 and 6.1 mJ/cm2 than for S. cerevisiae with 5.0 and 7.1 mJ/cm2, respectively. Irradiating with UVB, a higher irradiation dose for a reduction of 90% is needed for C. auris with 51.3 mJ/cm2 than for S. cerevisiae with 47.8 mJ/cm2.
Compared with both yeasts, a higher 1-log reduction dose was needed to inactivate C. cladosporioides for each wavelength with 21.2 mJ/cm2 at 222 nm, 44.1 mJ/cm2 at 254 nm, and 434.8 mJ/cm2 for UVB. The difference between the 1-log reduction doses increased with higher irradiation wavelengths.
Table 1 lists literature and this study’s experimental results for 1-log reduction doses of yeasts (various Candida species and S. cerevisiae) and spores of molds (C. cladosporioides, A. niger, and A. fumigatus) with respect to photoinactivation by different UV wavelengths (222, 254, and 302 nm).
Most data were found for irradiation at 254 nm. Furthermore, data could be retrieved for different representatives of the genus Candida. Most 1-log reduction doses for photoinactivation were published for C. albicans and C. auris.
Table 1. 1-Log Reduction Doses from Literature and From This Study (highlighted) in Liquid Media with Similar Experimental Conditions
|
Microorganism |
Wavelength (nm) |
1-Log reduction dose (mJ/cm2) |
k values (cm2/mJ) |
|
Candida albicans |
222 |
median value: 9.55 |
0.24 |
|
9.2 [30] 9.9 [31] |
0.25 0.23 |
||
|
254 |
median value: 11.8 |
0.20 |
|
|
11.8 [32] 7.6 [31] 6.5 [33] 16.0 [30] 17.0 [30] 19.0 [30] 22.0 [34] 9.7 [29] 8.3 [29] |
0.20 0.30 0.35 0.14 0.14 0.12 0.10 0.24 0.28 |
||
|
Candida auris |
222 |
4.3 (this study) |
0.54 (this study) |
|
254 |
median value: 17.5 |
0.13 |
|
|
6.1 (this study) 13.2 [29] 22.1 [29] 16.7 [29] 17.5 [29] 18.1 [29] 16.4 [29] 16.0 [29] 15.3 [29] 18.1 [29] 18.5 [29] 22.7 [35] 24.9 [35] |
0.38 (this study) 0.17 0.10 0.14 0.13 0.13 0.14 0.14 0.15 0.13 0.12 0.10 0.09 |
||
|
302 |
51.3 (this study) |
0.04 (this study) |
|
|
Candida kofuensis |
254 |
median value: 9.4 |
0.24 |
|
10.6 [36] 9.4 [36] 7.8 [36] |
0.22 0.24 0.30 |
||
|
Candida parapsilosis |
254 |
9.8 [37] |
0.23 |
|
Candida guilliermondii |
254 |
50.0 [38] |
0.05 |
|
302 |
5,000 (313 nm) [38] |
0.0005 |
|
|
Candida utilis |
254 |
80.0 [38] |
0.03 |
|
302 |
4,000 (313 nm) [38] |
0.0006 |
|
|
Saccharomyces cerevisiae |
222 |
5.0 (this study) |
0.46 (this study) |
|
254 |
median value: 7.1 |
0.32 |
|
|
7.1 (this study) 16.7 [39] 21.2 [40] 5.2 [41] 33.0 [42] 6.3 [43] 2.5 [44] |
0.32 (this study) 0.14 0.11 0.44 0.07 0.37 0.92 |
||
|
302 |
median value: 59.9 |
0.04 |
|
|
47.8 (this study) 72.0 [21] |
0.05 (this study) 0.03 |
||
|
Cladosporium |
222 |
21.2 (this study) |
0.11 (this study) |
|
254 |
median value: 233.4 |
||
|
44.1 (this study) 270.0 [34] 233.4 [45] |
0.05 (this study) 0.009 0.01 |
||
|
302 |
median value: 357.9 |
0.006 |
|
|
434.8 (this study) 281.0 [45] |
0.005 (this study) 0.008 |
||
|
Aspergillus niger spores |
222 |
median value: 85.0 |
0.03 |
|
106.8 [31] 72.5 [32] 85.0 [46] |
0.02 0.03 0.03 |
||
|
254 |
median value: 57.9 |
0.04 |
|
|
121.9 [31] 123.3 [46] 50.8 [32] 122.0 [47] 65.0 [48] 60.0 [49] 190.0 [34] 42.0 (265 nm) [48] 18.0 (280 nm) [48] 15.0 (280 nm) [49] |
0.02 0.02 0.05 0.02 0.04 0.04 0.01 0.05 0.13 0.15 |
||
|
Aspergillus fumigatus spores |
254 |
median value: 16.8 |
0.14 |
|
3.1 [50] 30.4 [51] |
0.74 0.08 |
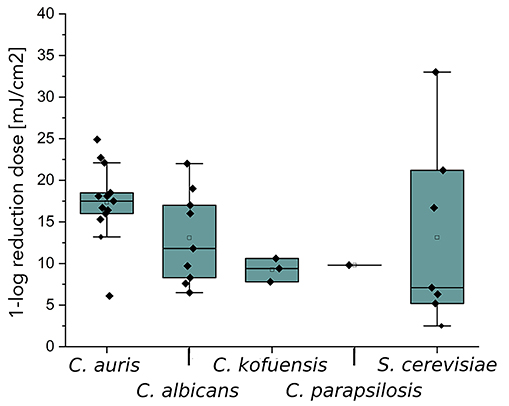
Figure 3 represents literature values and this study’s experimental results as a boxplot for irradiation at 254 nm. For C. albicans, the irradiation doses for a D90 reduction are very diverse and range from 6.5-22 mJ/cm2 and include reduction doses that are also needed for other Candida species, such as C. auris, C. davisiana, C. kofuensis, and C. parapsilopsis (Figure 3A). For C. guilliermondii and C. utilis, high 1-log reduction doses of 50.0 and 80.0 mJ/cm2 were determined [38]. For S. cerevisiae, 1-log reduction doses were measured and determined from literature sources, which range from 2.5-33.0 mJ/cm2.
For molds (Figure 3B), the most 1-log reduction doses were noted for A. niger, ranging from 15.0-190.0 mJ/cm2. Values from 44.1-270.0 mJ/cm2 formed the boxplot for C. cladosporioides. The 1-log reduction doses for A. fumigatus are 3.1- 30.4 mJ/cm2 for irradiation at 254 nm.
A.
B.
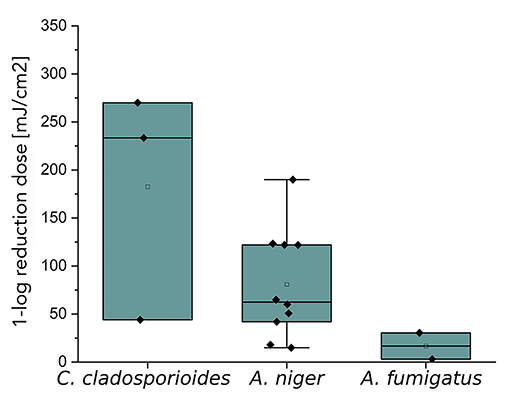
Figure 3. Representation of 1-log reduction doses with irradiation at 254 nm of different Candidaspecies as well as S. cerevisiae (A) and spores of molds like C. cladosporioides, A. niger, and A. fumigatus (B). The boxplots were created based on data (Table 3) from literature and measurements from this study.
Comparing the 1-log reduction doses in Table 3, most have been retrieved for irradiation at 254 nm. Comparable studies at other wavelength ranges, such as Far-UVC and UVB, were rare. In general, for all fungi and all irradiation wavelengths, exponential photoinactivation could be observed with rising 1-log reduction doses for higher wavelengths.
For 222 nm, a 1-log reduction dose of 4.3 mJ/cm2 was determined for C. auris in this study and is one of the first inactivation experiments at this wavelength. This dose is lower than the resulting median from various studies for C. albicans with a 1-log reduction dose of 9.55 mJ/cm2 at 222 nm in liquids. (Other studies focusing on surface disinfection using Far-UVC, like a recent investigation by Memic et al [52], were not included in the analysis, because of the incomparable experimental conditions.) For S. cerevisiae in suspension, a dose 1.2 times higher than for C. auris was determined with 5.0 mJ/cm2. When irradiated at 302 nm (broad-band UVB), very similar 1-log reduction doses of 51.3 and 47.8 mJ/cm2 were observed for C. auris and S. cerevisiae in liquid media, respectively (Figure 2).
In Table 1, the high D90 reduction doses at 254 nm with 50.0 and 80.0 mJ/cm2 of C. utilis and C. guilliermondii are particularly striking [38]. C. utilis is used in food industry and is classified as harmless [53–55]. C. guilliermondii has a low pathogenicity and can be found in biotechnology [56–58]. However, the amount of data from C. utilis and C. guilliermondii is too small to make reliable statements about a possible dependence of the pathogenicity. In comparison to other yeast, such as S. cerevisiae, which is also non-pathogenic, but for which similar 1-log reduction doses have been measured (Figure 2 and Figure 3), no dependence of pathogenicity on the irradiation dose can be demonstrated.
Figure 3 presents the literature and experimental 1-log reduction doses by irradiation at 254 nm. In Figure 3A, all literature values of C. auris are above the median value of C. albicans by a factor of 0.91. C. kofuensis and C. parapsilosis are below the median value of C. albicans. Furthermore, statements can be made about possible surrogates for C. auris and C. albicans. Since the 75% quantile of C. albicans is between the median and the 25% quantile of C. auris, C. auris could be described as a possible surrogate for C. albicans when irradiated at 254 nm. The box-forming values of S. cerevisiae are widely scattered and include all 1-log reduction doses of the other yeasts. However, the median and thus also the 25% quantile of the box is below the 25% quantile of the other yeasts. For this reason, S. cerevisiae is not a good surrogate for C. albicans for photoinactivation at 254 nm.
Both, Table 1 and Figure 3B reveal that much higher D90 doses are required for spores of the molds C. cladosporioides, A. niger, and A. fumigatus than for the yeasts. The 1-log reduction dose for C. cladosporioides with 434.8 mJ/cm2 is 10 times higher than for C. auris with 51.3 mJ/cm2 when irradiated at 302 nm. In Table 1, it is noticeable that hardly any data on photoinactivation are available for molds at the selected irradiation wavelengths. Especially for A. fumigatus, only 2 literature values are published for irradiation by UVC [50, 51]. No literature values are available for C. cladosporioides when irradiated by Far-UVC. In this study, a 1-log reduction dose of 21.2 mJ/cm2 was determined for this fungus, which is 4 times lower than the median value for A. niger with 85.0 mJ/cm2. For irradiation with UVB, only data for C. cladosporioides exists.
Figure 3B presents 1-log reduction doses from Table 1 as a boxplot for an irradiation at 254 nm. It is notable that the D90 values for all 3 molds differ from each other. The irradiation doses for A. niger and A. fumigatus are below the mean value and also partly below the measured value for C. cladosporioides. The recorded inactivation doses for C. cladosporioides are 44.1 mJ/cm2 up to 270.0 mJ/cm2 [34]. The median 1-log reduction dose for A. niger is 57.9 mJ/cm2 and the irradiation doses for A. fumigatus are 3.1 mJ/cm2 and 30.4 mJ/cm2 [50, 51]. For C. cladosporioides and A. niger, the median values for 1-log reduction doses differ by a factor of 2.7 at 254 nm. With regard to possible surrogate properties, this can be ruled out for A. niger on the aspect of photoinactivation at 222 nm of C. cladosporioides. This is also the case for irradiation at 254 for all 3 molds in relation to each other. For irradiation with UVB and irradiation at 222 nm with regard to A. fumigatus and C. cladosporioides, it is not possible to make a surrogate statement due to a lack of data.
There are various investigations for possible applications for the 3 wavelength ranges analyzed here. There is a broad spectrum of therapeutic approaches for various diseases (eg, skin diseases), possible disinfection systems and applications in the food industry [52, 59–61]. Even if many of these approaches can cure diseases or prevent infections in advance through disinfection, there are no clear statements to the question of how much the irradiation reduces the number of infections [62].
With regard to possible surrogates, the investigations carried out here described that S. cerevisiae represents a possible irradiation surrogate for C. auris for all 3 wavelengths. It was also possible to describe by comparing the literature and the resulting boxplot for irradiation at 254 nm that S. cerevisiae and C. kofuensis could be a surrogate for C. albicans for UVC irradiation experiments.
Due to the low number of photoinactivation data to be compared, further investigations should be performed on other fungi, both yeasts and molds. It is also important to carry out irradiation not only with UVC, but also with UVB and especially Far-UVC. Far-UVC has a great potential for application in the presence of people due to its low penetration depth and there are already methods for curing skin diseases with UVB. To obtain further information on possible surrogates or on the possible connection between pathogenicity and radiation sensitivity, more inactivation data must be available for the respective fungi.
The authors report no financial support for the research, authorship, and/or publication related to this article.
The authors report no competing financial interests.
1. World Health Organization. Antimicrobial Resistance Division; World Health Organization. Control of Neglected Tropical Diseases; World Health Organization. Global Coordination and Partnership; Alastruey-Izquierdo A. WHO fungal priority pathogens list to guide research, development and public health action; Organización Mundial de la Salud (OMS) 978-92-4-006025-8 2022.
2. Ahmad S, Asadzadeh M. Strategies to Prevent Transmission of Candida auris in Healthcare Settings. Curr Fungal Infect Rep. 2023;17(1):36-48. doi: 10.1007/s12281-023-00451-7. PubMed PMID: 36718372; PMCID: PMC9878498.
3. Du H, Bing J, Hu T, Ennis CL, Nobile CJ, Huang G. Candida auris: Epidemiology, biology, antifungal resistance, and virulence. PLoS Pathog. 2020;16(10):e1008921. doi: 10.1371/journal.ppat.1008921. PubMed PMID: 33091071; PMCID: PMC7581363.
4. Chakrabarti A, Singh S. Multidrug-resistant Candida auris: an epidemiological review. Expert Rev Anti Infect Ther. 2020;18(6):551-62. doi: 10.1080/14787210.2020.1750368. PubMed PMID: 32237924.
5. Dal Mas C, Rossato L, Shimizu T, Oliveira EB, da Silva Junior PI, Meis JF, Colombo AL, Hayashi MAF. Effects of the Natural Peptide Crotamine from a South American Rattlesnake on Candida auris, an Emergent Multidrug Antifungal Resistant Human Pathogen. Biomolecules. 2019;9(6). doi: 10.3390/biom9060205. PubMed PMID: 31141959; PMCID: PMC6627186.
6. Aldejohann AM, Martin R, Hecht J, Haller S, Rickerts V, Walther G, Eckmanns T, Kurzai O. Rise in Candida Auris Cases and First Nosocomial Transmissions in Germany. Dtsch Arztebl Int. 2023;120(27-28):447-78. doi: 10.3238/arztebl.m2023.0047. PubMed PMID: 37661316.
7. Vicente MF, Basilio A, Cabello A, Peláez F. Microbial natural products as a source of antifungals. Clin Microbiol Infect. 2003;9(1):15-32. doi: 10.1046/j.1469-0691.2003.00489.x. PubMed PMID: 12691539.
8. Horton MV, Nett JE. Candida auris infection and biofilm formation: going beyond the surface. Curr Clin Microbiol Rep. 2020;7(3):51-6. doi: 10.1007/s40588-020-00143-7. PubMed PMID: 33178552; PMCID: PMC7654955.
9. Adams E, Quinn M, Tsay S, Poirot E, Chaturvedi S, Southwick K, Greenko J, Fernandez R, Kallen A, Vallabhaneni S, Haley V, Hutton B, Blog D, Lutterloh E, Zucker H. Candida auris in Healthcare Facilities, New York, USA, 2013-2017. Emerg Infect Dis. 2018;24(10):1816-24. doi: 10.3201/eid2410.180649. PubMed PMID: 30226155; PMCID: PMC6154128.
10. Lockhart SR. Candida auris and multidrug resistance: Defining the new normal. Fungal Genet Biol. 2019;131:103243. doi: 10.1016/j.fgb.2019.103243. PubMed PMID: 31228646.
11. Kean R, Brown J, Gulmez D, Ware A, Ramage G. Candida auris: A Decade of Understanding of an Enigmatic Pathogenic Yeast. J Fungi (Basel). 2020;6(1). doi: 10.3390/jof6010030. PubMed PMID: 32110970; PMCID: PMC7150997.
12. Choi HI, An J, Hwang JJ, Moon SY, Son JS. Otomastoiditis caused by Candida auris: Case report and literature review. Mycoses. 2017;60(8):488-92. doi: 10.1111/myc.12617. PubMed PMID: 28378904.
13. Zatorska B, Moser D, Diab-Elschahawi M, Ebner J, Lusignani LS, Presterl E. The effectiveness of surface disinfectants and a micellic H(2)O(2) based water disinfectant on Candida auris. J Mycol Med. 2021;31(4):101178. doi: 10.1016/j.mycmed.2021.101178. PubMed PMID: 34388399.
14. Moore G, Schelenz S, Borman AM, Johnson EM, Brown CS. Yeasticidal activity of chemical disinfectants and antiseptics against Candida auris. J Hosp Infect. 2017;97(4):371-5. doi: 10.1016/j.jhin.2017.08.019. PubMed PMID: 28865738.
15. Cadnum JL, Shaikh AA, Piedrahita CT, Sankar T, Jencson AL, Larkin EL, Ghannoum MA, Donskey CJ. Effectiveness of Disinfectants Against Candida auris and Other Candida Species. Infect Control Hosp Epidemiol. 2017;38(10):1240-3. doi: 10.1017/ice.2017.162. PubMed PMID: 28793937.
16. Ramasamy K, Shanmugam M, Balupillai A, Govindhasamy K, Gunaseelan S, Muthusamy G, Robert BM, Nagarajan RP. Ultraviolet Radiation-induced Carcinogenesis: Mechanisms and Experimental Models. Journal of Radiation and Cancer Research. 2017;8(1):4-19. doi: 10.4103/0973-0168.199301. PubMed PMID: 02034728-201708010-00002.
17. Nishigori C, Yamano N, Kunisada M, Nishiaki-Sawada A, Ohashi H, Igarashi T. Biological Impact of Shorter Wavelength Ultraviolet Radiation-C(†). Photochem Photobiol. 2023;99(2):335-43. doi: 10.1111/php.13742. PubMed PMID: 36355343.
18. Harm W. Biological effects of ultraviolet radiation. United Kingdom: University Press; 1980.
19. Armstrong JD, Kunz BA. Photoreactivation implicates cyclobutane dimers as the major promutagenic UVB lesions in yeast. Mutat Res. 1992;268(1):83-94. doi: 10.1016/0027-5107(92)90086-h. PubMed PMID: 1378190.
20. Nascimento É, da Silva SH, Marques Edos R, Roberts DW, Braga GU. Quantification of cyclobutane pyrimidine dimers induced by UVB radiation in conidia of the fungi Aspergillus fumigatus, Aspergillus nidulans, Metarhizium acridum and Metarhizium robertsii. Photochem Photobiol. 2010;86(6):1259-66. doi: 10.1111/j.1751-1097.2010.00793.x. PubMed PMID: 20860693.
21. Fraĭkin G, Belenikina NS, Piniaskina EV, Rubin AB. [New photo-induced effects of reactivation and protection of yeast cells under lethal UVB radiation]. Izv Akad Nauk Ser Biol. 2013(6):754-9. PubMed PMID: 25518562.
22. Zwicker P, Schleusener J, Lohan SB, Busch L, Sicher C, Einfeldt S, Kneissl M, Kühl AA, Keck CM, Witzel C, Kramer A, Meinke MC. Application of 233 nm far-UVC LEDs for eradication of MRSA and MSSA and risk assessment on skin models. Sci Rep. 2022;12(1):2587. doi: 10.1038/s41598-022-06397-z. PubMed PMID: 35173210; PMCID: PMC8850561.
23. Buonanno M, Welch D, Brenner DJ. Exposure of Human Skin Models to KrCl Excimer Lamps: The Impact of Optical Filtering(†). Photochem Photobiol. 2021;97(3):517-23. doi: 10.1111/php.13383. PubMed PMID: 33465817; PMCID: PMC8247880.
24. Lopez-Malo A PE. Ultraviolet Light and Food Preservation. In: Barbosa-Cánovas GV, Tapia, M.S., Cano, M.P., editor. Novel food processing technologies: CRC Press; 2005. p. 405–21.
25. Hanamura N, Ohashi H, Morimoto Y, Igarashi T, Tabata Y. Viability evaluation of layered cell sheets after ultraviolet light irradiation of 222 nm. Regen Ther. 2020;14:344-51. doi: 10.1016/j.reth.2020.04.002. PubMed PMID: 32490060; PMCID: PMC7260610.
26. Araujo R, Rodrigues AG. Variability of germinative potential among pathogenic species of Aspergillus. J Clin Microbiol. 2004;42(9):4335-7. doi: 10.1128/jcm.42.9.4335-4337.2004. PubMed PMID: 15365039; PMCID: PMC516339.
27. Baker SE. Aspergillus niger genomics: past, present and into the future. Med Mycol. 2006;44 Suppl 1:S17-21. doi: 10.1080/13693780600921037. PubMed PMID: 17050415.
28. Li K, Zhu X, Qiao C, Zhang L, Gao W, Wang Y. The Gray Mold Spore Detection of Cucumber Based on Microscopic Image and Deep Learning. Plant Phenomics. 2023;5:0011. doi: 10.34133/plantphenomics.0011. PubMed PMID: 36930758; PMCID: PMC10013786.
29. Lemons AR, McClelland TL, Martin SB, Jr., Lindsley WG, Green BJ. Inactivation of the multi-drug resistant pathogen Candida auris using ultraviolet germicidal irradiation (UVGI). J Hosp Infect. 2020. doi: 10.1016/j.jhin.2020.04.011. PubMed PMID: 32283175; PMCID: PMC7748379.
30. Busbee DL, Sarachek A. Inactivation of Candida albicans by ultraviolet radiation. Arch Mikrobiol. 1969;64(4):289-314. doi: 10.1007/bf00417011. PubMed PMID: 5386170.
31. Clauss M SA, Hartung J. Ultraviolet disinfection with 222 nm wavelength—new options to inactivate UV-resistant pathogens. Proceedings of the 14th ISAH Congress: International Society for Animal Hygiene; 2009. p. 740-2.
32. Narita K, Asano K, Naito K, Ohashi H, Sasaki M, Morimoto Y, Igarashi T, Nakane A. 222-nm UVC inactivates a wide spectrum of microbial pathogens. J Hosp Infect. 2020. doi: 10.1016/j.jhin.2020.03.030. PubMed PMID: 32243946.
33. Dai T, Kharkwal GB, Zhao J, St Denis TG, Wu Q, Xia Y, Huang L, Sharma SK, d’Enfert C, Hamblin MR. Ultraviolet-C light for treatment of Candida albicans burn infection in mice. Photochem Photobiol. 2011;87(2):342-9. doi: 10.1111/j.1751-1097.2011.00886.x. PubMed PMID: 21208209; PMCID: PMC3048910.
34. Saprykina MN, Samsoni-Todorov AO, Todorov VV. The decontamination effect of UV radiation with respect to micromycetes. Journal of Water Chemistry and Technology. 2009;31(5):329-33. doi: 10.3103/S1063455X09050099.
35. Mariita RM, Davis JH, Lottridge MM, Randive RV. Shining light on multi-drug resistant Candida auris: Ultraviolet-C disinfection, wavelength sensitivity, and prevention of biofilm formation of an emerging yeast pathogen. Microbiologyopen. 2022;11(1):e1261. doi: 10.1002/mbo3.1261. PubMed PMID: 35212481; PMCID: PMC8767514.
36. Pereira VJ, Ricardo J, Galinha R, Benoliel MJ, Barreto Crespo MT. Occurrence and low pressure ultraviolet inactivation of yeasts in real water sources. Photochem Photobiol Sci. 2013;12(4):626-30. doi: 10.1039/c2pp25225b. PubMed PMID: 23001236.
37. Severin BF, Suidan MT, Engelbrecht RS. Kinetic modeling of U.V. disinfection of water. Water Research. 1983;17(11):1669-78. doi: 10.1016/0043-1354(83)90027-1.
38. Fraikin GY, Pospelov ME, Rubin LB. Repair of 313-NM induced lesions and photoprotection in yeast Candida guilliermondii. Photochem Photobiol. 1977;26(4):371-5. doi: 10.1111/j.1751-1097.1977.tb07499.x. PubMed PMID: 594172.
39. Azar Daryany MK, Massudi R, Hosseini M. Photoinactivation of Escherichia coli and Saccharomyces cerevisiae suspended in phosphate-buffered saline-A using 266- and 355-nm pulsed ultraviolet light. Curr Microbiol. 2008;56(5):423-8. doi: 10.1007/s00284-008-9110-3. PubMed PMID: 18259813.
40. Petin VG, Zhurakovskaya GP, Komarova LN. Fluence rate as a determinant of synergistic interaction under simultaneous action of UV light and mild heat in Saccharomyces cerevisiae. J Photochem Photobiol B. 1997;38(2-3):123-8. doi: 10.1016/s1011-1344(96)07449-0. PubMed PMID: 9203373.
41. Sommer R, Haider T, Cabaj A, Heidenreich E, Kundi M. Increased inactivation of Saccharomyces cerevisiae by protraction of UV irradiation. Applied and Environmental Microbiology. 1996;62(6):1977-83. doi: 10.1128/aem.62.6.1977-1983.1996.
42. Kim JK, Petin VG, Tkhabisimova MD. Survival and recovery of yeast cells after simultaneous treatment of UV light radiation and heat. Photochem Photobiol. 2004;79(4):349-55. doi: 10.1562/2003-11-21-ra.1. PubMed PMID: 15137512.
43. Watanabe M, Masaki H, Mori T, Tsuchiya T, Konuma H, Hara-Kudo Y, Takatori K. Inactivation effects of UV irradiation and ozone treatment on the yeast and the mold in mineral water. J Food Prot. 2010;73(8):1537-42. doi: 10.4315/0362-028x-73.8.1537. PubMed PMID: 20819369.
44. Kiefer J. The effect of caffeine on survival of UV-irradiated diploid yeast strains of different sensitivities. Mutat Res. 1975;30(3):317-26. PubMed PMID: 1105164.
45. Latorre BA, Rojas S, Díaz GA, Chuaqui H. Germicidal effect of UV light on epiphytic fungi isolated from blueberry. Ciencia E Investigacion Agraria. 2012;39(3):473-80. doi: 10.4067/s0718-16202012000300007. PubMed PMID: WOS:000313400900007.
46. Clauß M. Higher effectiveness of photoinactivation of bacterial spores, UV resistant vegetative bacteria and mold spores with 222 nm compared to 254 nm wavelength. Acta hydrochimica et hydrobiologica. 2006;34(6):525-32. doi: 10.1002/aheh.200600650.
47. Taylor-Edmonds L, Lichi T, Rotstein-Mayer A, Mamane H. The impact of dose, irradiance and growth conditions on Aspergillus niger (renamed A. brasiliensis) spores low-pressure (LP) UV inactivation. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2015;50(4):341-7. doi: 10.1080/10934529.2015.987519. PubMed PMID: 25723059.
48. Wan Q, Wen G, Cao R, Zhao H, Xu X, Xia Y, Wu G, Lin W, Wang J, Huang T. Simultaneously enhance the inactivation and inhibit the photoreactivation of fungal spores by the combination of UV-LEDs and chlorine: Kinetics and mechanisms. Water Res. 2020;184:116143. doi: 10.1016/j.watres.2020.116143. PubMed PMID: 32688151.
49. Wan Q, Wen G, Cao R, Xu X, Zhao H, Li K, Wang J, Huang T. Comparison of UV-LEDs and LPUV on inactivation and subsequent reactivation of waterborne fungal spores. Water Res. 2020;173:115553. doi: 10.1016/j.watres.2020.115553. PubMed PMID: 32028247.
50. Nourmoradi H, Nikaeen M, Stensvold CR, Mirhendi H. Ultraviolet irradiation: An effective inactivation method of Aspergillus spp. in water for the control of waterborne nosocomial aspergillosis. Water Res. 2012;46(18):5935-40. doi: 10.1016/j.watres.2012.08.015. PubMed PMID: 22985523.
51. Sisti M, Schiavano GF, Santi M, Brandi G. Ultraviolet germicidal irradiation in tap water contaminated by Aspergillus spp. J Prev Med Hyg. 2017;58(4):E315-e9. doi: 10.15167/2421-4248/jpmh2017.58.4.777. PubMed PMID: 29707663; PMCID: PMC5912791.
52. Memic S, Kaple CE, Cadnum JL, Donskey CJ. Evaluation of an Automated Wall-mounted Far Ultraviolet-C Light Technology for Continuous or Intermittent Decontamination of Candida auris on Surfaces. Pathog Immun. 2024;9(1):156-67. doi: 10.20411/pai.v9i1.683. PubMed PMID: 38779368; PMCID: PMC11110956.
53. Scoppettuolo G, Donato C, De Carolis E, Vella A, Vaccaro L, La Greca A, Fantoni M. Candida utilis catheter-related bloodstream infection. Med Mycol Case Rep. 2014;6:70-2. doi: 10.1016/j.mmcr.2014.10.003. PubMed PMID: 25473600; PMCID: PMC4246400.
54. Buerth C, Heilmann CJ, Klis FM, de Koster CG, Ernst JF, Tielker D. Growth-dependent secretome of Candida utilis. Microbiology (Reading). 2011;157(Pt 9):2493-503. doi: 10.1099/mic.0.049320-0. PubMed PMID: 21680638.
55. Buerth C, Tielker D, Ernst JF. Candida utilis and Cyberlindnera (Pichia) jadinii: yeast relatives with expanding applications. Appl Microbiol Biotechnol. 2016;100(16):6981-90. doi: 10.1007/s00253-016-7700-8. PubMed PMID: 27357226.
56. Papon N, Savini V, Lanoue A, Simkin AJ, Crèche J, Giglioli-Guivarc’h N, Clastre M, Courdavault V, Sibirny AA. Candida guilliermondii: biotechnological applications, perspectives for biological control, emerging clinical importance and recent advances in genetics. Curr Genet. 2013;59(3):73-90. doi: 10.1007/s00294-013-0391-0. PubMed PMID: 23616192.
57. Savini V, Catavitello C, Onofrillo D, Masciarelli G, Astolfi D, Balbinot A, Febbo F, D’Amario C, D’Antonio D. What do we know about Candida guilliermondii? A voyage throughout past and current literature about this emerging yeast. Mycoses. 2011;54(5):434-41. doi: 10.1111/j.1439-0507.2010.01960.x. PubMed PMID: 21039941.
58. Regina Alvares Da Silva Lira I, Mendes Da Silva Santos E, Paredes Selva Filho AA, Bronzo Barbosa Farias C, Medeiros Campos Guerra J, Asfora Sarubbo L, Moura De Luna J. - Biosurfactant Production from Candida Guilliermondii and Evaluation of Its Toxicity2020.
59. Singh H, Bhardwaj SK, Khatri M, Kim K-H, Bhardwaj N. UVC radiation for food safety: An emerging technology for the microbial disinfection of food products. Chemical Engineering Journal. 2021;417:128084. doi: 10.1016/j.cej.2020.128084.
60. Jeong YJ, Ha JW. Simultaneous Effects of UV-A and UV-B Irradiation on the Survival of Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria monocytogenes in Buffer Solution and Apple Juice. J Food Prot. 2019;82(12):2065-70. doi: 10.4315/0362-028x.Jfp-19-131. PubMed PMID: 31714805.
61. Myers E, Kheradmand S, Miller R. An Update on Narrowband Ultraviolet B Therapy for the Treatment of Skin Diseases. Cureus. 2021;13(11):e19182. doi: 10.7759/cureus.19182. PubMed PMID: 34873522; PMCID: PMC8634827.
62. Schleusener J, Lohan SB, Busch L, Ghoreschi K, Ploch NL, May S, Vogel S, Eberle J, Meinke MC. Treatment of the Candida subspecies Candida albicans and Candida parapsilosis with two far-UVC sources to minimise mycoses in clinical practice. Mycoses. 2023;66(1):25-8. doi: 10.1111/myc.13521. PubMed PMID: 35986595.
Submitted June 10, 2024 | Accepted July 31, 2024 | Published September 4, 2024
Copyright © 2024 The Authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License.