
Samir Memic1,2, Claire E. Kaple3, Jennifer L. Cadnum2, and Curtis J. Donskey4,5
1Department of Systems Biology, Case Western Reserve University School of Medicine, Cleveland, Ohio
2Research Service, Louis Stokes Cleveland VA Medical Center, Cleveland, Ohio
3Department of Molecular Biology and Microbiology, Case Western Reserve University School of Medicine, Cleveland, Ohio
4Department of Medicine, Case Western Reserve University School of Medicine, Cleveland, Ohio
5Geriatric Research, Education, and Clinical Center, Louis Stokes Cleveland VA Medical Center, Cleveland, Ohio
Curtis J. Donskey
Curtis.Donskey@va.gov
Memic S, Kaple KE, Cadnum JL, Donskey CJ. Evaluation of an Automated Wall-mounted Far Ultraviolet-C Light Technology for Continuous or Intermittent Decontamination of Candida auris on Surfaces. Pathogens and Immunity. 2024;9(1):156–167. doi: 10.20411/pai.v9i1.683
10.20411/pai.v9i1.683
Background: Technologies that provides safe and effective decontamination of surfaces and equipment between episodes of manual cleaning could be an important advance in efforts to prevent transmission of the emerging fungal pathogen Candida auris.
Methods: We tested the efficacy of a novel wall-mounted far ultraviolet-C (UV-C) light technology that delivers far UV-C, when people are not detected within the field of illumination, against C. auris isolates from clades I, II, III, and IV using a quantitative disk carrier test method. In an equipment room, we examined the efficacy of the technology in reducing an isolate of C. auris from clade IV inoculated on multiple sites on portable devices.
Results: The far UV-C technology reduced isolates from all 4 clades of C. auris by >3 log10 colony-forming units (CFU) after an 8-hour exposure on steel disks. For the clade IV isolate, similar reductions were achieved on glass and plastic carriers. In the equipment room, the technology reduced C. auris inoculated on multiple sites on portable equipment by >2 log10 CFU in 4 hours.
Conclusions: The far UV-C technology could be useful for decontamination of surfaces and equipment between episodes of manual cleaning. Additional studies are needed to evaluate the use of the technology in clinical settings.
Far ultraviolet-C; environment; Candida auris; portable equipment
Candida auris is a globally emerging multidrug-resistant fungal pathogen that is classified as an urgent threat by the Centers for Disease Control and Prevention [1]. In the United States, C. auris cases have increased steadily in recent years with documented spread to 28 states and Washington DC by the end of 2022 [2]. Contaminated surfaces and reusable medical equipment have been implicated as important sources of transmission [3–6]. Therefore, thorough cleaning and disinfection of surfaces in patients’ rooms at least daily and of shared equipment after each use is recommended [1]. However, adequate cleaning and disinfection is challenging because personal items and patient care equipment are often present in occupied patient rooms, and surfaces near colonized patients rapidly become re-contaminated after disinfection (ie, within 4 hours) [7].
A technology that provides safe and effective decontamination of surfaces and equipment between episodes of manual cleaning could be an important advance in efforts to prevent transmission of C. auris. One promising candidate technology is far ultraviolet-C (UV-C) light (200 to 230 nm) [8]. Far UV-C light is strongly absorbed by proteins and other biomolecules, resulting in minimal penetration into skin or eye tissues [8]. There is growing evidence from animal model and human volunteer studies that far UV-C doses within threshold limit values proposed by the American Conference of Governmental Industrial Hygienists and the International Commission on Non-Ionizing Radiation Protection may be safe [8–13]. We previously demonstrated that far UV-C was effective against C. auris isolates from clades II and III; although, the reductions were relatively modest in comparison to vegetative bacterial pathogens (ie, 0.5 to 2.9 log10 colony-forming unit reductions in 45 minutes versus >3 log10 CFU reductions for vegetative bacteria) [14]. Here, we conducted a more comprehensive evaluation of the efficacy of a far UV-C technology against strains from all 4 clades of C. auris. The technology tested is novel in that it detects people and can be programmed to stop or reduce far UV-C delivery when people are present within the area of exposure.
The far UV-C technology (Myna Life Technologies, Inc.) uses 3 krypton-chloride excimer lamps that emit a primary wavelength of 222 nm with filters to block emitted wavelengths >230 nm [14]. Figure 1 shows a picture of the device. Each device contains 3 lamps with a field of illumination of 60° per lamp [14]. Two wall-mounted devices are recommended for a typical patient room or equipment room. For this study, the devices were mounted on posts. The device includes proprietary sensors that detect people within the field of illumination, including individuals remaining motionless. For this study, the device was programmed to automatically discontinue all far UV-C light delivery when people enter the field of illumination, to stay off while people are present, and to resume output 30 seconds after people exit the field of illumination. The study protocol was approved by the Research and Development and Biosafety Committees at the Louis Stokes Cleveland VA Medical Center (protocol number 1584025).

Figure 1. Picture of the wall-mounted far ultraviolet-C device.
The C. auris test strains included isolates from 4 phylogenetic clades, including AR-0381 (Clade II; East Asia origin), AR-0389 (Clade I; South Asia origin), AR-0383 (Clade III; Africa origin), and AR-0385 (Clade IV; South America origin). Isolates from 4 clades were studied because there are potential differences in susceptibility to UV-C light. In previous studies, C. auris isolates from clades III and IV (AR-0383 and AR-0385) formed aggregates and exhibited reduced susceptibility to 254 nm UV-C light in comparison to a clade II isolate (AR-0381) [15, 16].
For comparison, we tested Candida albicans American Type Culture Collection (ATCC) strain 10231, Candida glabrata clinical strain MRL#9547, and a clinical Candida parapsilosis strain from the Cleveland VA Medical Center.
Testing was conducted in a 25.6 m3 room. Two devices were positioned at opposite corners of one wall, 2 m from the floor, angled toward the center of the room, with 1.6 m of space between the devices. With all 6 lamps from the 2 devices operating continuously, the calculated doses of far UV-C delivered over 8 hours at 2 and 3 m from the lamps were 230 and 150 mJ/cm2, respectively [14].
We tested the efficacy of the technology against the test organisms using a modification of the American Society for Testing and Materials (ASTM) standard quantitative disk carrier test method (ASTM E 2197-02) and standard practice for determining antimicrobial efficacy of ultraviolet germicidal irradiation against microorganisms on carriers with simulated soil [17, 18]. A soil load of 5% fetal calf serum was used. A 10 µL inoculum containing ~4 log10 CFU of the test organism was spread to cover 20 mm steel disks. The disks were oriented horizontally 0.8 m high. All experiments were completed in triplicate. Disks were processed as previously described [17]. Log10 reductions were calculated in comparison to untreated controls. For testing on carriers, a 3 log10 or greater reduction in the test organisms in comparison to untreated controls was considered effective [14].
An initial set of experiments was conducted with the C. auris clade I isolate to assess effectiveness over time at different distances from the devices. The inoculated disks were placed 1, 2, or 3 m from the devices. Exposure times of 0.75, 4, 8, 24, and 72 hours were tested. A radiometer (UIT2400 Handheld Light Meter for 222 nm, Ushio America) was used to measure irradiance at 1, 2, and 3 m from the devices. Based on the initial results, all the remaining organisms were tested at 2 m from the devices with exposure times of 4, 24, and 72 hours.
We determined the inactivation rate constants for the test isolates using a modification of the methods of Lemons et al [19] and Vitzilaiou et al [20]. A 5 mL suspension containing ~106 andida species CFU per mL water was added to a petri dish with a magnetic stirrer set at 200 rpm. The suspension was exposed to increasing doses of far UV-C delivered by a single far UV-C device. The doses were 0, 25, 50, 75, 100, 125, 150, and 175 mJ/cm2. Following exposure, aliquots of the suspension were plated on selective media to quantify Candida species as previously described [17]. Experiments were repeated twice with triplicate samples for each test point. A dose-response curve was plotted by graphing the survival fraction at each far UV-C dose. The inactivation rate constants (k-values) were calculated as previously described [19, 20]. The k-value is inversely related to the dose required to obtain a specific survival fraction.
The clade IV isolate was selected for subsequent testing because the Environmental Protection Agency (EPA) recommends the use of C. auris AR #0385 (clade IV) as the test strain for testing the efficacy of disinfectants [21]. To assess the impact of different types of surfaces on the efficacy of far UV-C, experiments were conducted with the AR #0385 isolate inoculated onto steel disks, plastic coupons (acrylonitrile butadiene styrene polymer), and glass slides. The carriers were placed 2 m from the devices with exposure times of 0.75 and 4 hours.
For these experiments, testing was conducted in a 35.2 m3 equipment storage room. Two devices were placed on opposite sides of the room at a height of 2 m. The devices were placed on opposite sides of the room to reduce the potential for shaded areas to receive suboptimal far UV-C dosing. A workstation-on-wheels, portable vital signs unit, and wheelchair were inoculated on 2.5 cm diameter circular areas with 10 µL containing 104 CFU of C. auris AR #0385 in 5% fetal calf serum; 2 to 3 sites were inoculated for each type of equipment including sites on the side that would not be in direct line of sight of the far UV-C light. The test sites ranged from 1.5 to 2.2 m from the nearest device. After 0.75 and 4 hours of exposure, the inoculated sites were sampled with pre-moistened cotton-tipped swabs. The swabs were processed to quantify C. auris as previously described [3]. Testing was completed in triplicate. Log10 reductions were calculated in comparison to untreated control surfaces. For inoculated equipment, a 2 log10 or greater reduction compared with untreated control surfaces was deemed effective as an adjunct to standard cleaning and disinfection.
To assess the feature that discontinues far UV delivery when people are within the area of far UV-C exposure, research personnel entered the equipment room 10 times while the device was operating, stood with minimal motion at multiple locations in the vicinity of the equipment for 5 minutes, then exited the room. Personnel determined if the device was on or off based on visual assessment (ie, the lamps emit visible light when operating) and by carrying the handheld radiometer to measure irradiance.
A one-factor analysis of variance (ANOVA) was used to test for significant differences in k-values among the different Candida species isolates. A one-way ANOVA was used to compare the log10 reductions for C. auris clade IV on steel, plastic, and glass carriers. Data were analyzed using R version 3.5.0 software (The R Foundation for Statistical Computing, Vienna, Austria).
As shown in Figure 2, the clade I isolate was reduced by >2 log10 CFU after 0.75 hours of exposure at 1, 2, and 3 m from the far UV-C devices. After 8 hours of exposure, no C. auris was recovered at 1, 2, or 3 m. Irradiance readings at 1, 2, and 3 m from the devices were 9.0, 9.4, and 5.8 µW/cm2. All the remaining test organisms were reduced by >3 log10 CFU after 8 hours and to undetectable levels after exposure for 24 and 72 hours of exposure at 2 m from the devices.
Figure 3 shows the far UV-C dose-response curves and the inactivation rate constants (k-values) for each of the Candida species isolates. The survival fraction at each far UV-C dose is represented by black dots, and the red dashed lines represent the best-fit line using the exponential decay model. There were no statistically significant differences in the k-values among the Candida species tested (P=0.44; one-factor ANOVA). The k-values for the C. auris isolates ranged from 0.114 to 0.165. There was no substantial reduction in any of the Candida species isolates for controls not exposed to far UV-C.
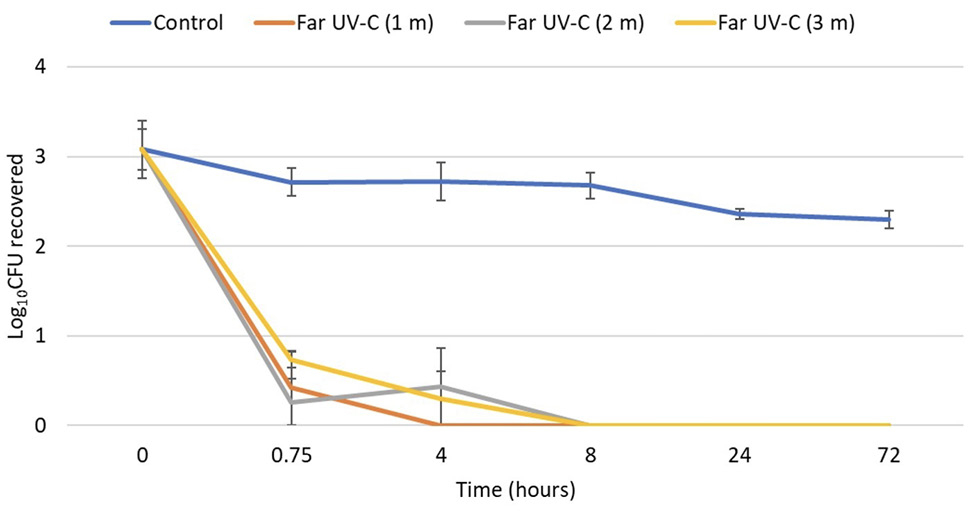
Figure 2. Efficacy of 2 far ultraviolet-C (UV-C) devices in reducing a clade I isolate of Candida auris on steel disk carriers at 1, 2, and 3 m from the devices over 72 hours. The 2 devices were positioned at opposite corners of one wall 2 m from the floor, angled toward the center of the room, with 1.6 m of space between the devices. Control carriers were unexposed to far UV-C. CFU, colony-forming unit.
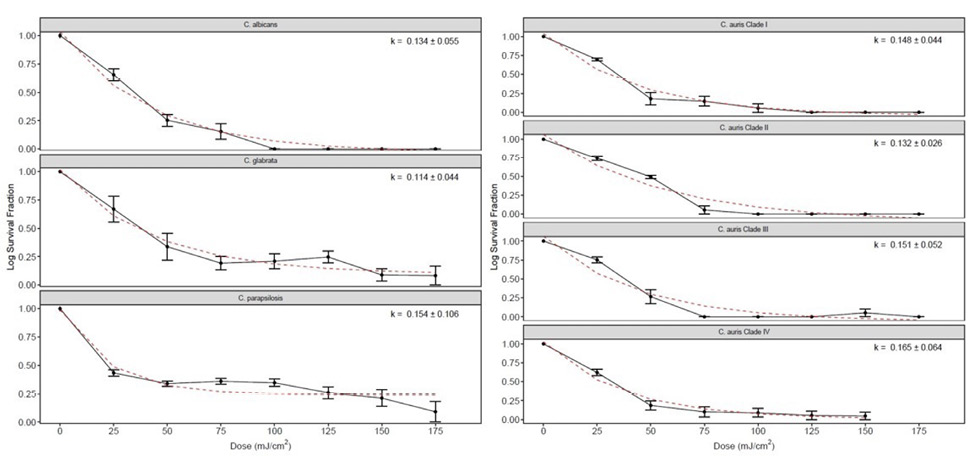
Figure 3. Far ultraviolet-C (UV-C) dose-response curves and the inactivation rate constants (k-values + SE) for each of the Candida species isolates. The survival fraction at each far UV-C dose is represented by black dots. The red dashed lines represent the best-fit line using the exponential decay model. There were no statistically significant differences in the k-values among the Candida species tested (P=0.44).
Figure 4 shows a comparison of the log10 CFU reductions of the C. auris clade IV isolate on steel, plastic, and glass carriers. There were no significant differences in the reductions achieved with each type of carrier after 0.75 and 4 hours of far UV-C exposure (P>0.05).
Figure 5 shows the efficacy of the far UV-C technology in reducing the C. auris clade IV isolate on a workstation-on-wheels, portable vital signs unit, and wheelchair in an equipment room. C. auris was reduced by >2 log10 CFU on all inoculated sites after 4 hours of exposure.
The far UV-C devices in the equipment room consistently turned off when research personnel walked into the areas of far UV-C exposure and remained off while they stood still at multiple locations in the vicinity of the portable equipment. The devices turned back on 30 seconds after personnel exited the area of far UV-C delivery. Based on irradiance readings, there was no substantial exposure to far UV-C light (ie, consistent readings of 0 µW/cm2) during entries into the room.
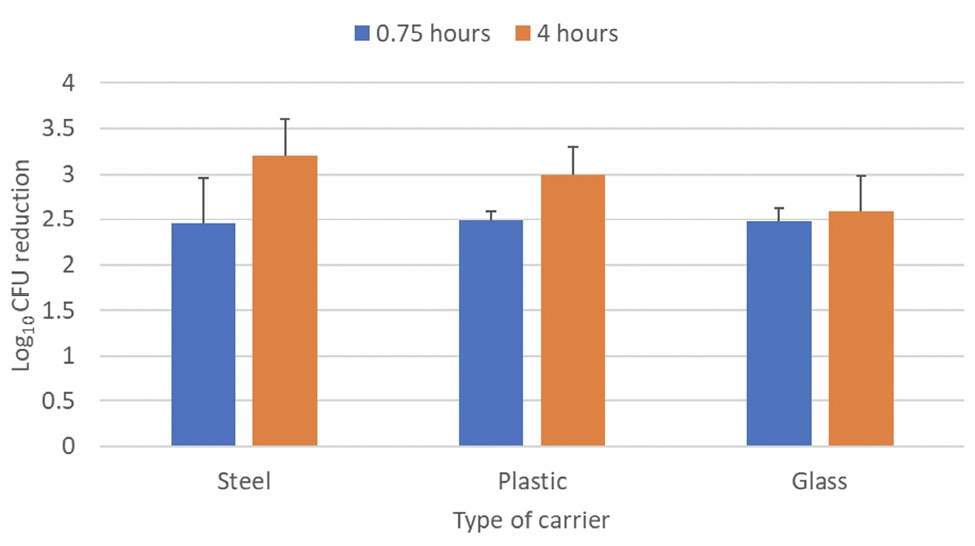
Figure 4. Efficacy of 2 far ultraviolet-C (UV-C) devices in reducing a clade I isolate of Candida aurison steel disk, plastic, and glass carriers. The carriers were placed 2 m from the devices and exposed to far UV-C for 0.75 or 4 hours. Control carriers were unexposed to far UV-C. CFU, colony-forming unit.
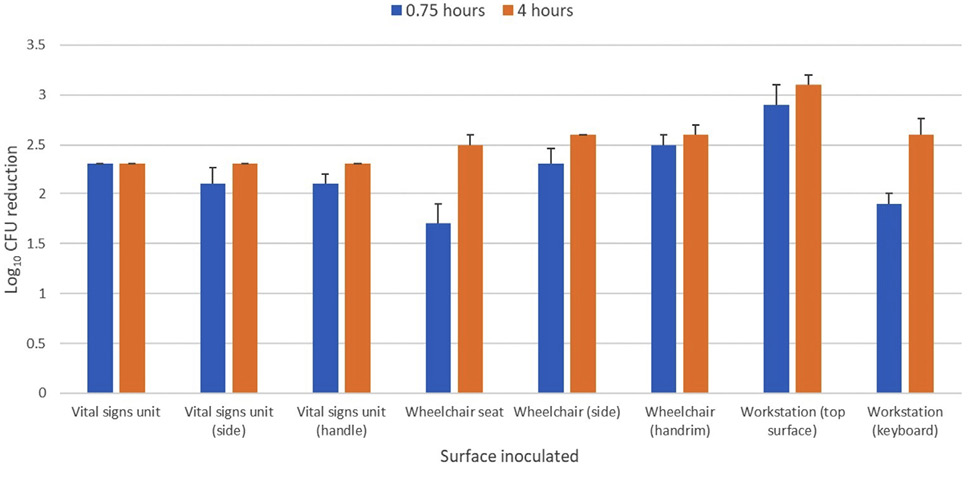
Figure 5. Efficacy of 2 far ultraviolet-C (UV-C) devices in reducing a clade IV isolate of Candida auris inoculated on portable medical devices in an equipment room. The 2 devices were placed on opposite sides of the room at a height of 2 m. The exposure time was 0.75 and 4 hours and test sites ranged from 1.5 to 2.2 m from the nearest device. Control sites were unexposed to far UV-C. CFU, colony-forming unit.
We found that an automated wall-mounted far UV-C technology was effective in reducing isolates from the 4 major clades of C. auris by >3 log10 CFU after an 8-hour exposure on steel disks and to undetectable levels after 24 and 72 hours of exposure. The k-values of the C. auris isolates for far UV-C ranged from 0.114 to 0.165. These k-values are equivalent to k-values previously reported for the same C. auris isolates from the 4 major clades for 254 nm UV-C light (range, 0.130 to 0.176) [19]. For the clade IV isolate, similar reductions were achieved on glass and plastic carriers in 4 hours. In an equipment room, the technology reduced the clade IV C. auris isolate on multiple sites inoculated on real-world equipment by >2 log10 CFU in 4 hours. These findings suggest that the far UV-C technology could be a useful addition to current approaches to address environmental contamination with C. auris.
Safety is an important concern for all UV-C technologies [9]. For the current study, the technology was modified to provide automated delivery of far UV-C only when people were not present. This modification would provide adequate far UV-C doses in areas such as portable equipment rooms that are occupied infrequently or procedure or clinic rooms that may be unoccupied for several minutes between patients. In contrast to standard 254 nm UV-C, if accidental short-term exposure to far UV-C did occur, there would be relatively little risk because such exposure would be below the 8-hour threshold limit values proposed for far UV-C exposure (161 mJ/cm2 for eyes and 479 mJ/cm2 for skin) [8]. For patient rooms or other frequently occupied areas, the device can be programmed to automatically reduce or discontinue far-UV-C exposure by turning off one or more light modules as needed based on the proximity of people to keep exposure below a threshold limit value of 160 mJ/cm2 per 8 hours [8]. Prior to considering routine implementation of far UV-C in occupied areas, additional evaluations of long-term safety are needed. Such evaluations are currently being conducted in clinical settings.
Our study has some limitations. First, we evaluated efficacy on carriers and portable equipment inoculated with C. auris. Additional studies are needed in real-world settings where contamination is due to shedding by colonized patients. Second, only 45-minute and 4-hour exposures were tested in the equipment room. Longer exposure times would be anticipated in equipment rooms that are occupied infrequently. Third, it is not known if the reductions in contamination that were achieved will be sufficient to reduce the risk for transmission of C. auris. Fourth, although we focused on C. auris, patients are often co-colonized with C. auris and other healthcare-associated pathogens [7]. In previous studies, far UV-C light has been shown to be effective against other healthcare-associated pathogens [14]. Finally, we did not compare the efficacy of the far UV-C technology with other potential technologies that might provide continuous decontamination of surfaces [9]. Products such as continuously active quaternary ammonium disinfectants may also be effective in reducing C. auris, but may have limitations (eg, easily removed by wiping, efficacy may vary with method of application) [22, 23].
In summary, our findings suggest that the far UV-C technology we studied could be useful for decontamination of surfaces and equipment between episodes of manual cleaning. Given that C. auris is classified as an urgent threat, there is an urgent need for additional studies to evaluate the use of the technology in clinical settings.
C.J.D has received research grants from Clorox and Pfizer. All other authors report no conflicts of interest relevant to this article.
This work was supported by the Department of Veterans Affairs.
1. Candida auris: Centers for Disease Control and Prevention; 2023 [updated September 26, 2023; cited 2024 February 11]. Available from: https://www.cdc.gov/fungal/candida-auris/index.html.
2. Lyman M, Forsberg K, Sexton DJ, Chow NA, Lockhart SR, Jackson BR, Chiller T. Worsening Spread of Candida auris in the United States, 2019 to 2021. Ann Intern Med. 2023;176(4):489-95. doi: 10.7326/m22-3469. PubMed PMID: 36940442.
3. Piedrahita CT, Cadnum JL, Jencson AL, Shaikh AA, Ghannoum MA, Donskey CJ. Environmental Surfaces in Healthcare Facilities are a Potential Source for Transmission of Candida auris and Other Candida Species. Infect Control Hosp Epidemiol. 2017;38(9):1107-9. doi: 10.1017/ice.2017.127. PubMed PMID: 28693657.
4. Eyre DW, Sheppard AE, Madder H, Moir I, Moroney R, Quan TP, Griffiths D, George S, Butcher L, Morgan M, Newnham R, Sunderland M, Clarke T, Foster D, Hoffman P, Borman AM, Johnson EM, Moore G, Brown CS, Walker AS, Peto TEA, Crook DW, Jeffery KJM. A Candida auris Outbreak and Its Control in an Intensive Care Setting. N Engl J Med. 2018;379(14):1322-31. doi: 10.1056/NEJMoa1714373. PubMed PMID: 30281988.
5. Haq MF, Pearlmutter BS, Cadnum JL, Donskey CJ. Efficacy of 23 commonly used liquid disinfectants against Candida auris isolates from the 4 major clades. Infect Control Hosp Epidemiol. 2024;45(1):127-31. doi: 10.1017/ice.2023.157. PubMed PMID: 37528766.
6. Kumar J, Eilertson B, Cadnum JL, Whitlow CS, Jencson AL, Safdar N, Krein SL, Tanner WD, Mayer J, Samore MH, Donskey CJ. Environmental Contamination with Candida Species in Multiple Hospitals Including a Tertiary Care Hospital with a Candida auris Outbreak. Pathog Immun. 2019;4(2):260-70. doi: 10.20411/pai.v4i2.291. PubMed PMID: 31768483; PMCID: PMC6827507.
7. Sansom SE, Gussin GM, Schoeny M, Singh RD, Adil H, Bell P, Benson EC, Bittencourt CE, Black S, Del Mar Villanueva Guzman M, Froilan MC, Fukuda C, Barsegyan K, Gough E, Lyman M, Makhija J, Marron S, Mikhail L, Noble-Wang J, Pacilli M, Pedroza R, Saavedra R, Sexton DJ, Shimabukuro J, Thotapalli L, Zahn M, Huang SS, Hayden MK. Rapid Environmental Contamination with Candida auris and Multidrug-Resistant Bacterial Pathogens Near Colonized Patients. Clin Infect Dis. 2023. doi: 10.1093/cid/ciad752. PubMed PMID: 38059527.
8. Blatchley Iii ER, Brenner DJ, Claus H, Cowan TE, Linden KG, Liu Y, Mao T, Park S-J, Piper PJ, Simons RM, Sliney DH. Far UV-C radiation: An emerging tool for pandemic control. Critical Reviews in Environmental Science and Technology. 2023;53(6):733-53. doi: 10.1080/10643389.2022.2084315.
9. Donskey CJ. Continuous surface and air decontamination technologies: Current concepts and controversies. Am J Infect Control. 2023;51(11s):A144-a50. doi: 10.1016/j.ajic.2023.03.017. PubMed PMID: 37890945.
10. Hessling M, Haag R, Sieber N, Vatter P. The impact of far-UVC radiation (200-230 nm) on pathogens, cells, skin, and eyes - a collection and analysis of a hundred years of data. GMS Hyg Infect Control. 2021;16:Doc07. doi: 10.3205/dgkh000378. PubMed PMID: 33643774; PMCID: PMC7894148.
11. Welch D, Aquino de Muro M, Buonanno M, Brenner DJ. Wavelength-dependent DNA Photodamage in a 3-D human Skin Model over the Far-UVC and Germicidal UVC Wavelength Ranges from 215 to 255 nm. Photochem Photobiol. 2022;98(5):1167-71. doi: 10.1111/php.13602. PubMed PMID: 35104367; PMCID: PMC9544172.
12. Sugihara K, Kaidzu S, Sasaki M, Ichioka S, Takayanagi Y, Shimizu H, Sano I, Hara K, Tanito M. One-year Ocular Safety Observation of Workers and Estimations of Microorganism Inactivation Efficacy in the Room Irradiated with 222-nm Far Ultraviolet-C Lamps. Photochem Photobiol. 2023;99(3):967-74. doi: 10.1111/php.13710. PubMed PMID: 36081379.
13. Kousha O, O’Mahoney P, Hammond R, Wood K, Eadie E. 222 nm Far-UVC from filtered Krypton-Chloride excimer lamps does not cause eye irritation when deployed in a simulated office environment. Photochem Photobiol. 2024;100(1):137-45. doi: 10.1111/php.13805. PubMed PMID: 37029739; PMCID: PMC10952573.
14. Memic S, Osborne AO, Cadnum JL, Donskey CJ. Efficacy of a far-ultraviolet-C light technology for continuous decontamination of air and surfaces. Infect Control Hosp Epidemiol. 2024;45(1):132-4. doi: 10.1017/ice.2023.159. PubMed PMID: 37529841.
15. Chatterjee P, Choi H, Ochoa B, Garmon G, Coppin JD, Allton Y, Lukey J, Williams MD, Navarathna D, Jinadatha C. Clade-specific variation in susceptibility of Candida auris to broad-spectrum ultraviolet C light (UV-C). Infect Control Hosp Epidemiol. 2020;41(12):1384-7. doi: 10.1017/ice.2020.410. PubMed PMID: 33046172; PMCID: PMC7720409.
16. Pearlmutter BS, Haq MF, Cadnum JL, Jencson AL, Carlisle M, Donskey CJ. Efficacy of relatively low-cost ultraviolet-C light devices against Candida auris. Infect Control Hosp Epidemiol. 2022;43(6):747-51. doi: 10.1017/ice.2021.206. PubMed PMID: 34011417.
17. Standard Quantitative Disk Carrier Test Method for Determining Bactericidal, Virucidal, Fungicidal, Mycobactericidal, and Sporicidal Activities of Chemicals. West Conshohocken, PA: ASTM International; 2011.
18. Standard practice for determining antimicrobial efficacy of ultraviolet germicidal irradiation against microorganisms on carriers with simulated soil. West Conshohocken, PA: ASTM International; 2018.
19. Lemons AR, McClelland TL, Martin SB, Jr., Lindsley WG, Green BJ. Inactivation of the multi-drug resistant pathogen Candida auris using ultraviolet germicidal irradiation (UVGI). J Hosp Infect. 2020. doi: 10.1016/j.jhin.2020.04.011. PubMed PMID: 32283175; PMCID: PMC7748379.
20. Vitzilaiou E, Kuria AM, Siegumfeldt H, Rasmussen MA, Knøchel S. The impact of bacterial cell aggregation on UV inactivation kinetics. Water Res. 2021;204:117593. doi: 10.1016/j.watres.2021.117593. PubMed PMID: 34482094.
21. Standard Operating Procedure for Quantitative Method for Evaluating the Efficacy of Antimicrobial Products against Candida auris on Hard, Non-Porous Surfaces. SOP Number: MB-35-03: US Environmental Protection Agency Office of Pesticide Programs; [updated October 12, 2021]. Available from: https://www.epa.gov/system/files/documents/2021-10/mb-35-03.pdf.
22. Redmond SN, Cadnum JL, Silva SY, Pearlmutter BS, Jencson AL, Alhmidi H, Wilson BM, Donskey CJ. Evaluation of a continuously active disinfectant for decontamination of portable medical equipment. Infect Control Hosp Epidemiol. 2022;43(3):387-9. doi: 10.1017/ice.2021.66. PubMed PMID: 34034834; PMCID: PMC8961336.
23. Cadnum JL, Memic S, Jencson AL, Donskey CJ. Why is there a discrepancy between laboratory test results and real-world efficacy of continuously active quaternary ammonium disinfectants? Infect Control Hosp Epidemiol. 2024:1-3. doi: 10.1017/ice.2024.15. PubMed PMID: 38343341.
Submitted February 12, 2024 | Accepted May 8, 2024 | Published May 17, 2024
Copyright © 2024 The Authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License.