Irem Karaman1*, Asmita Pathak2*, Defne Bayik2, Dionysios C. Watson2
1Bahcesehir University School of Medicine, Istanbul, Turkey
2Sylvester Comprehensive Cancer Center, Miller School of Medicine, University of Miami, Florida
*These authors contributed equally
Dionysios C. Watson
dionysios.watson@miami.edu
Karaman I, Pathak A, Bayik D, Watson DC. Harnessing Bacterial Extracellular Vesicle Immune Effects for Cancer Therapy. Pathogens and Immunity. 2024;9(1):56–90. doi:10.20411/pai.v9i1.657
10.20411/pai.v9i1.657
There are a growing number of studies linking the composition of the human microbiome to disease states and treatment responses, especially in the context of cancer. This has raised significant interest in developing microbes and microbial products as cancer immunotherapeutics that mimic or recapitulate the beneficial effects of host-microbe interactions. Bacterial extracellular vesicles (bEVs) are nano-sized, membrane-bound particles secreted by essentially all bacteria species and contain a diverse bioactive cargo of the producing cell. They have a fundamental role in facilitating interactions among cells of the same species, different microbial species, and even with multicellular host organisms in the context of colonization (microbiome) and infection. The interaction of bEVs with the immune system has been studied extensively in the context of infection and suggests that bEV effects depend largely on the producing species. They thus provide functional diversity, while also being nonreplicative, having inherent cell-targeting qualities, and potentially overcoming natural barriers. These characteristics make them highly appealing for development as cancer immunotherapeutics. Both natively secreted and engineered bEVs are now being investigated for their application as immunotherapeutics, vaccines, drug delivery vehicles, and combinations of the above, with promising early results. This suggests that both the intrinsic immunomodulatory properties of bEVs and their ability to be modified could be harnessed for the development of next-generation microbe-inspired therapies. Nonetheless, there remain major outstanding questions regarding how the observed preclinical effectiveness will translate from murine models to primates, and humans in particular. Moreover, research into the pharmacology, toxicology, and mass manufacturing of this potential novel therapeutic platform is still at early stages. In this review, we highlight the breadth of bEV interactions with host cells, focusing on immunologic effects as the main mechanism of action of bEVs currently in preclinical development. We review the literature on ongoing efforts to develop natively secreted and engineered bEVs from a variety of bacterial species for cancer therapy and finally discuss efforts to overcome outstanding challenges that remain for clinical translation.
bacterial extracellular vesicles; cancer immunotherapy; bioengineering; biomarker; vaccine; drug delivery; targeting; drug development; clinical translation
The human microbiome consists of the bacteria, fungi, viruses, archaea, and protozoans that symbiotically co-exist in and on the body [1, 2]. Commensal microbes have been detected in increasing anatomic locations outside the prototypical microbiome of the gut, including skin [3], lungs [4], genito-urinary tract [5], and blood [6]. As an integral part of organism homeostasis, the microbiome impacts endocrine function [7, 8], cardiovascular function [9], immune regulation [10], nutrient digestion [11], and even drug metabolism [12]. As a result, microbiome dysregulation, or dysbiosis, is being associated with a wide variety of diseases and clinical outcomes, including cancer [13, 14]. Following early preclinical results suggesting that an intact microbiome is critical to immunotherapy responsiveness [15], clinical studies demonstrated that the specific composition of the microbiome is associated with response and resistance to immune checkpoint inhibitor therapy across several cancer types [16–18]. Moreover, healthy donor fecal microbiome transplantation combined with PD-1 inhibition demonstrated efficacy in melanoma patients refractory to PD-1 inhibitor monotherapy [19, 20].
The microbiome’s effect on cancer immunotherapy highlights the promise of developing microbiome-based therapeutics and their value as potential biomarkers. However, the limited understanding of mechanisms that regulate host-microbiome interactions remains a challenge, despite increasing numbers of studies showing a correlation between the composition of the gut microbiome and clinical outcomes. Akkermansia mucinophila [21], Bifidobacterium longum [22], Ruminococcaceae [16], and a high Firmicute-to-Bacteroidia ratio [16, 23] have all been linked to checkpoint inhibitor response in various cancers. One proposed mechanism for these observations is the effect of microbe-derived secreted metabolites on immunity upon absorption through the gut mucosa [24]. For instance, acetate produced by Bifidobacterium bifidum [25] and the short-chain fatty acid butyrate [26] were shown to enhance anti-tumor immunity by increasing tumor-infiltrating IFNγ secreting CD8+ T cells. In addition, a series of studies has identified microbes within solid tumors, which are associated with immunotherapy response [27–29]. While there has been some debate [30] regarding the validity of studies that rely on sequencing data to characterize tumor microbiomes [31], several studies have employed orthogonal approaches (including histological analyses and culturing of live tumor-derived bacteria) to support the presence of viable microbes within tumors [27, 29]. As understanding of the rules governing tumor microbe colonization and the molecular basis of their effects increases, opportunities may arise for relevant diagnostics and therapeutics in this area as well.
Besides metabolites and viable bacterial cells, bacterial extracellular vesicles (bEVs) comprise an additional bioactive component of the microbiome. These nano-sized (~20 to 200 nm) vesicular structures contain biologically active macromolecules (lipids, glycans, proteins, nucleic acids) and are produced by essentially all bacteria [32–34]. The role of microbiome-derived bEVs in modulating immunity in cancer is only just beginning to be explored, with most studies focusing on effects bEVs have on cells in the lamina propria of the gastrointestinal tract [35–37]. Proof-of-principle studies have demonstrated that bEVs administered systemically can modulate the immune microenvironment of tumors and affect treatment response [38–40]. These studies have raised significant interest in bEVs as potential therapeutics and biomarkers.
In this review, we first present an overview of established literature describing the immune effects of bEVs in various disease contexts and at barrier sites, as data suggests that this comprises the primary mechanism of action of therapeutic bEVs. We then discuss how bEVs are being developed as potential cancer therapeutics in preclinical studies. Future studies on the mechanisms governing bEV-host interactions will be instrumental in the development of relevant biomarkers and therapeutics.
Ongoing research suggests that the generation of bEVs is a tightly controlled mechanism characterized by the inclusion of specific biologically active components [41]. These bEVs serve as vectors for bacteria-bacteria and bacteria-host communication by interacting with recipient cell receptors, delivering biological macromolecules, and incorporating them into the host cell cytoplasm [33]. Through these interactions, bEVs mediate intracellular signaling, horizontal gene transfer, virulence factor delivery [11, 42–45], and immune modulation [46]. Three primary mechanisms for the effects of bEVs have been suggested: endocytosis, membrane fusion, and receptor-mediated signaling. The kinetics of bEV-host cell entrance is governed by species-specific composition, such as the presence of specific structures of lipopolysaccharides (LPS) in gram-negative bEVs [11, 47–49]. The bEVs from some species, such as those produced by Aggregatibacter actinomycetemcomitans, have also been shown to be internalized by cells and sorted to a peri-nuclear localization through clathrin-mediated endocytosis [50]. On the other hand, bEVs with O-antigens, such as those produced by Escherichia coli, can enter via lipid raft-dependent endocytosis subsequent to toll-like receptor (TLR) recognition [49].
Until recently, research on bEVs has mostly concentrated on outer membrane vesicles generated by gram-negative bacteria by membrane blebbing. Gram-positive bacteria, which lack an outer membrane, were initially thought likely to be incapable of EV release due to their strong peptidoglycan cell walls [51]. A renewed focus on the formation of these vesicles has been sparked by the discovery of physiologically active EVs in gram-positive bacteria in more recent investigations [51–53]. Structural variations between gram-negative and gram-positive bacteria induce distinct EV biogenesis pathways. Three main theories have been put forward to explain how gram-negative bacteria release extracellular vesicles: genetic mutations that reduce the strength of the outer membrane-peptidoglycan bond, stress responses that cause the accumulation of envelope components, and changes in the stability of the outer membrane caused by interactions with LPS [46]. Gram-positive bacteria form vesicles by budding through the cytoplasmic membrane and entering the cell wall. The synthesis of EVs in gram-positive bacteria is assumed to be influenced by factors such as penicillin-binding proteins (PBPs) and autolysins, as well as genetic factors. As illustrated by Wang et al (2018) [53], specific mutations in penicillin-binding proteins and autolysin have been observed to impact EV production, quantity, and size in Staphylococcus aureus, where EV release is correlated with the degree of peptidoglycan cross-linking. This provides more evidence that gram-positive bacterial species may exhibit substantial variation in the EV production process.
In-depth proteomic and biochemical analyses have revealed that bEVs, like their parent bacteria, transport a variety of cargo, including immunostimulatory ligands such as membrane-bound and periplasmic pathogen-associated molecular patterns (PAMPs) [54], enzymes, toxins [55], polysaccharides [56], nucleic acids (DNA and RNA) [57], LPS, lipoteichoic acid (LTA) [58], and peptidoglycan [59]. Both commensal and pathogenic bEVs have been shown to modulate the host immune system via PAMPs that are recognized by host pattern recognition receptors (PRRs) such as TLRs and nucleotide-binding oligomerization domain-containing protein 1 (NOD1) [11]. The different biogenesis pathways during vesiculogenesis, the distinctive membrane envelope structure of their parental bacterium, the genetic make-up of the producing strain, and growth conditions all contribute to the heterogeneity of bEVs in terms of their structure, size, density, and molecular cargo composition. These properties in return have implications on vesicle binding, kinetics in tissues, immunologic effects, and other biological functions [60].
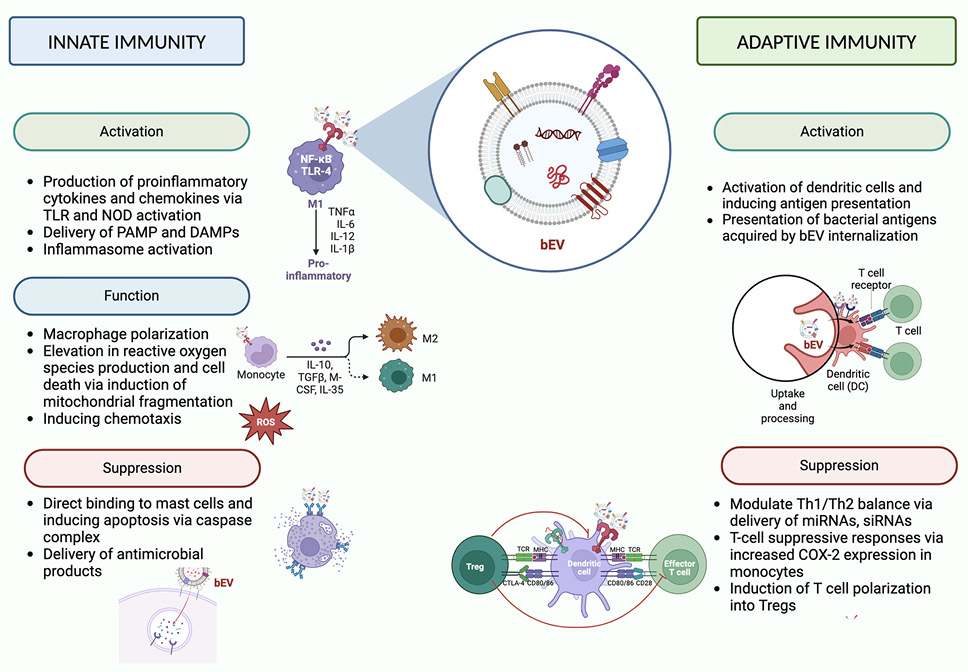
While there are some broadly-described mechanisms of bEV-host interactions (eg, NFκB activation via LPS/TLR4 [61] and LTA/TLR2 interactions [62]), the complete spectrum of their molecular interactions and associated downstream effects depend on the producing bacteria strain, the recipient cell identity, and the biological context of the interaction (summarized in Figure 1). For example, it has been reported that E. coli bEVs elicit the production of TLR4-mediated CXCL8 [76], while Lactobacillus casei [78] and Lactobacillus fermentum [78] LTA in bEVs stimulates the production of proinflammatory cytokines and chemokines in a TLR2-dependent manner. Helicobacter pylori bEVs activate cellular peptidoglycan sensor NOD1, whereas Pseudomonas aeruginosa bEVs generate atypical inflammation in human monocytes and mouse macrophages by inflammasome activation, IL-1 production, and cell death via caspase-5 [63, 64]. In addition, bEVs have also been shown to play a role in macrophage polarization. Chen et al reported that Fusobacterium nucleatum bEVs aggravated periodontitis by macrophage polarization towards a pro-inflammatory phenotype [65].

Figure 1. Effects of bacterial extracellular vesicles on innate and adaptive immunity. Graphical depictions represent examples of effects attributed to bEVs. DAMPs (damage-associated molecular patterns). Created with BioRender.com.
In contrast, Mycobaterium tuberculosis bEVs suppress the immune response by activation of immunosuppressive macrophages [66]. T-cell suppressive responses are also modulated by H. pylori bEVs via increased COX-2 expression in monocytes [63]. Lactobacillus bEVs can attenuate LPS-stimulated inflammatory signals in macrophages and microglial cells downstream of Erk and p38 signaling pathways. A similar observation was noted using murine macrophages by Spirometra erinaceieuropaei plerocercoid bEVs [67]. These and other studies demonstrate the large breadth of downstream effects that bEVs can mediate on the immune system.
An increasing number of studies are interrogating the role of bEVs at barrier sites, such as lungs, intestine, and sinuses, in the context of infection. For example, bEVs in bronchoalveolar lavage fluid activate cytokines and inflammatory mediators in alveolar macrophages [68], while various strains of Enterovirus bEVs are associated with acute gastroenteritis [69]. The bEVs in nasal lavage fluid activate mucin-type O-glycan biosynthesis and the TGFb pathway in chronic rhinosinusitis [70]. Acinetobacter baumannii bEVs are taken up by mammalian cells and activate the host GTPase dynamin-related protein 1 that enhances its accumulation on mitochondria, causes mitochondrial fragmentation, elevation in reactive oxygen species production, and cell death [71].
In addition, bEVs have been shown to be involved in non-infectious diseases, such as autoimmuninty, asthma, and atopic dermatitis. For instance, Akkermansia muciniphila bEVs demonstrated protective effects on the dextran sulfate sodium (DSS)-induced model of inflammatory bowel disease (IBD) [72], suggesting bEVs as a potential mediator of commensal microbe-host interaction in this disease. In another study of this IBD model, orally administered bEVs from Bacteroides thetaiotaomicron also ameliorated inflammation [73]. In addition to gastrointestinal microbiota, alterations in lung microbiota have been shown to affect mucosal immunity. For example, Pseudomonas aeruginosa has been shown to secrete regulatory sRNA52320, a fragment of a P. aeruginosa methionine tRNA that has been loaded into bEVs, reducing LPS-induced IL-8 secretion in cultured primary human airway epithelial cells [74]. Using metagenomic analysis, increased levels of the bEVs of Klebsiella spp and decreased levels of bEVs from Lactobacillus, Sphingomonas, Akkermansia, and Micrococcus spp were found in the serum of patients with asthma [75, 76]. Lactococcus lactis bEVs modulate T helper (Th)1/Th2 balance by stimulating dendritic cells in a mouse model of S. aureus-induced atopic dermatitis [77]. Lactobacillus plantarum EVs demonstrated protection against S. aureus-induced atopic dermatitis [78]. Bifidobacterium bEVs have been shown to be associated with asthma susceptibility and atopic dermatitis by binding to mast cells and suppressing the allergic reaction inducing mast cell apoptosis [79].
In summary, bEVs from diverse microbial sources have been shown to have potent effects on immunity in a variety of contexts. These intrinsic properties have led to significant interest in the development of bEVs as a therapeutic immunomodulation platform, including for cancer treatment, as discussed below.
While cancer immunotherapy has transformed the treatment of individuals with cancer, not all patients benefit from these approaches [80]. Dysfunctional immune activation and/or redundant mechanisms of anti-tumor immunity exhaustion in the immunosuppressive tumor microenvironment (TME) play fundamental roles in facilitating cancer immune evasion [81]. Thus, strategies that boost immune activity in the TME can be useful to overcome resistance to immunotherapy [82].
Initial efforts to harness microbes for cancer treatment included developing oncolytic bacteria and bacteria expressing cytotoxic proteins or tumor-specific antigens [83, 84]. Accumulating research has demonstrated that several bacterial species can potentially eliminate tumors by direct bacterial cytotoxicity, enhanced host immune response, and production of enzymes, bacteriocins, and toxins that disrupt the proliferation of cancer cells [85]. Attenuated Salmonella typhimurium and Clostridium novy, which were modified to express HlyE, Stx2, and recA, resulted in stimulation of the host immune response, leading to the activation of cytokines such as IL-2, IL-4, IL-18, and CCL21, ultimately resulting in tumor regression and necrosis in preclinical models of various solid tumors [86, 87]. However, phase 1 clinical trials have failed to show tumor shrinkage in patients with metastatic melanoma, squamous cell carcinoma, and other solid tumors [86, 87]. Mice treated with Escherichia coli or Salmonella typhimurium strains producing the ClyA toxin were shown in a small number of trials to have reduced tumor development [88, 89]. Nevertheless, several critical obstacles remain in clinical applications of bacteriotherapy in cancer treatment, including bacterial persistence, toxicity, DNA instability, and targeting efficiency [84].
As described in the previous section, bEVs possess immunomodulatory effects that are dependent on the microbial source and target immune cell, which constitute them as cell-free alternatives to bacteriotherapy. Given that essentially all bacteria produce them, bEVs exhibit wide heterogeneity in terms of dimensions, surface elements, and molecular cargo, making this a versatile platform technology for therapeutic development [90]. In addition, bEVs have the capability to traverse biological barriers while maintaining stability in the bloodstream [91, 92]. They also have the potential to deliver their payload with some selectivity to the TME, either by passive accumulation as biological nanoparticles [40, 93], by engineered expression of tumor-targeting ligands [93], or by incorporation into host cells that home to tumors [94]. Furthermore, there is some successful clinical experience with bEVs, exemplified by the MenBVac vaccine for Neisseria meningitidis [95]. As a result of these characteristics, harnessing the immunostimulatory properties of bEVs along with additional engineering (such as incorporation of chemotherapeutic drugs and tumor-targeting ligands) comprises an area of intense research efforts [96–98].
While efforts to develop therapeutic bEVs for cancer have utilized a diverse pool of source bacterial strains (summarized in Table 1), many have focused on E. coli strains given the availability of relevant reagents and ease of modification. In an early study of bEVs as a potential immunotherapeutic intervention for cancer treatment, Kim et al [40] utilized bEVs derived from E. coli that had been genetically engineered to lack the msbB gene, resulting in a strain with reduced endotoxin activity.
The authors demonstrated bEV accumulation in subcutaneous mouse tumor models and the capacity to elicit a durable anti-cancer immune response that leads to substantial reductions in tumor sizes, dependent on CD8+ cells and the presence of IFN-γ [40]. In another study, E. coli BL21 (DE3) bEVs effectively induced immune responses, mediated by IL-6, TNF-α, IFN- γ, and CXCL10, which eliminated subcutaneous tumors in mouse models of urothelial and breast carcinoma [99]. Additionally, that study revealed that the administration of bEVs resulted in an increase in the population of intratumoral CD8+ T cells with stem-like properties that specifically target cancer antigens. This augmentation rendered the cancer cells more receptive to the effects of anti-PD-1 antibody immunotherapy. In addition, Won et al have shown that the application of E. coli bEVs in the TME resulted in the infiltration of T lymphocytes that specifically target cancer cells and express exhaustion markers, such as PD-1, TIM3, and CD39 [99], suggesting the potential for combination with checkpoint inhibitors that are in clinical use or under development.
Table 1. Engineered bEVs for Therapeutic Applications in Cancer
|
bEV source |
Engineering method |
Treatment model |
Treatment effect |
Reference |
|
Loading |
||||
|
E. coli |
Synthetic bEVs generated with lysozyme and high pH treatment, resulting in bEVs with fewer cytosolic components |
Melanoma (B16F10) or colon carinoma (CT26 cells)‐bearing mice |
Tumor growth inhibition, enhanced efficiency of anti-PD1 therapy |
[100] |
|
E. coli BL21 |
Functionalizing bEVs with tumor-targeting DNA aptamers |
4T1 breast cancer-bearing mice |
Selective pyroptosis, increased effector T-cell infiltration, decreased regulatory T cells, suppression of tumor growth |
[101] |
|
E. coli Nissle 1917 |
Perhexiline-loaded E. coli bEVs |
CT26 colon cancer cell line in vitro |
Repolarization of macrophages to M1 phenotype, apoptosis and inhibition of cell growth, invasion and migration |
[102] |
|
Klebsiella pneumonia |
Doxorubicin-loaded bEVs |
A549 lung tumor-bearing BALB/c nude mice |
Tumor growth inhibition, recruitment of macrophages into tumor microenvironment |
[103] |
|
E. coli BL21(ΔmsbB) |
bEVs loaded with paclitaxel and Redd1 siRNA |
Triple-negative breast cancer in vivo model (4T1 tumor-bearing mice) |
Enhancement of glycolysis in M2 macrophages with polarization, shift in tumor metabolism, suppression of tumor growth, inhibit tumor metastasis |
[104] |
|
Targeting ligands |
||||
|
E. coli |
bEVs expressing an EGFR-targeting scFv version of panitumumab |
Triple-negative breast cancer murine models (4T1tumor bearing mice) |
Enhanced tumor targeting, increased M1 macrophages and CD8 T lymphocytes |
[105] |
|
E. coli strain W3110 |
bEVs expressing ectodomain of programmed death 1 (PD1) |
C57BL/6 mice bearing B16 melanoma cells, Balb/c mice bearing CT26 colorectal cancer cells |
Recruitment of cytotoxic T cells and dereased immune inhibitory PD-1/PD-L1 effects, enhanced anti-tumor response |
[106] |
|
Combination of loading and targeting |
||||
|
E. coli K-12 W3110 strain |
bEV-associated HER2-specific antibody in the surface by fusion to Cytolysin A, and loaded with siRNA targeting kinesin spindle protein |
Cell lines that overexpress HER2 (SKOV3, BT474, and HCC-1954), HCC-1954 xenografts |
Suppressed tumor proliferation in vitro and in vivo |
[107] |
|
Attenuated Salmonella typhimurium |
bEV coating with DSPE-PEG-RGD and loaded with 5-fluorouracil(5-FU) prodrug tegafur |
B16F10 melanoma, 4T1 breast cancer models in vivo |
Inhibition of tumor growth, decreased metastatic nodules to lungs in melanoma model. |
[109] |
|
bEV source |
Engineering method |
Treatment model |
Treatment effect |
Reference |
|
Magnetospirillum gryphiswaldense |
bEVs containing iron ions, doxorubicin and modified with DSPE-PEG-cRGD peptides |
4T1 breast cancer and drug-resistant MCF-7/ADR tumors in mice |
Inhibition of tumor gowth and metastasis to the lung in a 4T1-Luc model of breast cancer metastasis to the lung in vivo, ferroptosis of targeted cancer cells |
[109] |
|
E. coli |
miRNA nano-delivery system using zeolitic imidazolate framework-8 (ZIF-8) coated with bEVs that are engineered to display PD1 |
Murine breast cancer model |
Increased tumor treatment efficiency in murine breast cancer models in vivo by enhancing immune activation and checkpoint inhibition mediated by bEV-PD1 |
[110] |
|
Salmonella typhimurium-pGFlaB |
Doxorubicin-loaded Salmonella bEVs |
Mice bearing C6 glioma |
Inhibition of tumor growth, macrophage repolarization, neutrophil recruitment, P-gp downregulation |
[94] |
In addition, the investigation of the application of bEVs from other species is ongoing. Akkermansia muciniphila bEVs were shown to have anti-tumor effects in a syngeneic prostate cancer mouse model by increasing the CD8+ lymphocytes and promoting macrophage differentiation into an inflammatory phenotype [111]. Bacterial EVs from other gram-negative strains (Salmonella enterica) and gram-positive sources (Lactobacillus acidophilus and Staphylococcus aureus) also showed anti-cancer effects in mice with CT26 tumors [40]. In addition, bEVs derived from Bacillus licheniformisin inhibited the tumor growth both in MDA-MB-231 breast cancer and A549 lung cancer cell lines [112]. These studies suggest that diverse speceies’ bEVs could have beneficial immunomodulatory properties for cancer therapy.
Despite these promising results, there is still the concern that bEV engagement of TLRs will lead to severe systemic toxicity. Some groups have approached this challenge by modifying the parental bacteria to attenuate endotoxin levels in bEVs [40, 107]. Others performed additional processing of purified bEVs with high pH treatment resulting in lower quantities of bacterial protein, RNA, and DNA [100]. While these bEVs exhibited an inability to induce an immunologic response through TLR3, 7, 8, and 9, they retained their ability to serve as adjuvants for a mouse melanoma-derived EV-based vaccine, suppressing tumor growth and metastasis and enhancing the treatment effect of anti-PD-1 antibodies [100]. Yet another approach has been to shield endotoxin with tumor-targeting DNA aptamers. In one application, this enabled triggering LPS-dependent pyroptosis in preclinical breast cancer models without severe systemic toxicity [101].
Direct experimental evidence in cancer models or extrapolation from microbiology and infectious disease research suggests that the anti-tumor effects of bEVs are largely driven by interaction between bEV components with innate immune receptors. However, there is also evidence that bEVs can directly impact cancer cell biology. For instance, Akkermansia muciniphila bEVs can transfer bacterial acetyltransferase to both colorectal cancer cell lines and mouse cancer models, resulting in increased H3K14 acetylation and heat-shock protein 70 (Hsp70) expression, which indirectly induces immune activation of CD8+ cytotoxic T lymphocytes [113]. The bEVs produced by E. coli strain A5922 were shown to induce oxidative stress, increase PINK1 expression, and reduce mitochondrial membrane potential in HT-29 colon cancer cells, which led to decreased viability [92]. In HepG2 hepatic cancer cells, Lactobacillus rhamnosus GG bEVs also resulted in a higher Bax/Bcl-2 ratio with activation of apoptosis and subsequent cell death in vitro [114]. Direct effects on cancer cells may also synergize with cancer-directed therapies, as was the case with Klebsiella pneumoniae bEVs that enhanced the anti-cancer properties of tamoxifen in MCF-7 cells via the activation of Cyclin E2 and pERK signaling, which resulted in inhibition of cancer cell proliferation in vitro [115].
Despite being considered potential innovative therapeutic tools, there are several questions regarding the optimal dose, delivery method, biodistribution, and pharmacokinetics of bEVs. Until now, the majority of pre-clinical investigations have focused on examining the biodistribution and pharmacokinetics of EVs through the utilization of mouse models. Various biodistribution studies demonstrated that bEVs are primarily found in organs with a dense vascular network and other tissues connected to the reticuloendothelial system, such as liver, spleen, lung, and kidneys [116]. Furthermore, in the case of mammalian EVs, half-life in blood varies by cell source, ranging from 30 minutes to 6 hours before being cleared by reticuloendothelial macrophages, which detect negatively charged phosphatidylserine on the membrane of EVs [117–119]. Thus, it is possible that the source species will also impact the pharmacokinetics of bEVs. The impact of the administration method on the circulation time and biodistribution of bEVs remains poorly comprehended, as only a limited number of research projects have explicitly evaluated different administration routes, mostly utilizing mammalian cell-derived EVs administered in mice [120]. Although the animal studies offer useful insights into the biodistribution of EVs, these findings may not be completely applicable to bEVs. Variations in extracellular protein/lipid composition, EV labeling technique, and the type of EV donor cells may all have a role in EV biodistribution beyond the initial route of delivery. Circulation times may also be influenced by species distinctions between the recipient and the EV source [121].
Given the rapid elimination of bEVs from circulation, substantial research is needed to design optimal bEV therapeutics able to reach target tissues. One such approach involves the direct delivery of bEVs to tumors for local and/or abscopal therapeutic effects [122]. Additional strategies may include transient blockade of rapid clearance by the reticuloendothelial system, previously shown to enhance tumor accumulation of administered mammalian EVs [119]. Finally, applications of bEVs from some species may have more favorable pharmacokinetic profiles than others. As described in the section below, a different approach could involve engineering to alter the intrinsic properties of bEVs. This area of research is far more advanced in the field of mammalian EVs and synthetic nanoparticles [123–126], which could provide significant insight for bEV applications as well. For example, mammalian EV circulating time was significantly increased when they were engineered to express the “don’t-eat-me” signal, CD47, which inhibits phagocytosis by macrophages upon ligation with its receptor, SIRPα [127]. Others have modified the surface of EVs and nanoparticles by chemical modification (click chemistry), glycan modification, and insertion of specific peptides to further modify their pharmacokinetic characteristics [128]. In summary, combinations of optimized administration and engineering strategies are likely to be needed for the effective application of bEVs in cancer therapy.
Engineering bEVs for therapeutic applications in cancer. Aside from having inherent biological activity, bEVs can be further modified to construct customized drug delivery systems either by engineering the producing bacteria or isolated bEVs.
Various methodologies have been devised to encapsulate exogenous proteins and pharmaceutical agents into bEVs, often applying technologies developed for synthetic nanoparticles. These approaches have demonstrated the ability of bEVs to acquire supplementary biological attributes [129]. Escherichia coli Nissle 1917 bEVs passively loaded with perhexiline delivered this compound to macrophages, which were reprogrammed from an immunosuppressive to an inflammatory phenotype [102]. When these reprogrammed macrophages were co-cultured with CT26 colon cancer cells, they promoted apoptosis and inhibited invasion and migration [102]; bEVs generated from attenuated Klebsiella pneumonia passively loaded with doxorubicin also induced cell apoptosis, cytotoxicity, and immunogenicity by attracting macrophages in the TME of mice with a transplantable model of non-small-cell lung cancer [103]. In a mouse model of triple-negative breast cancer, bEVs from E. coli BL21 were surface-functionalized with paclitaxel and electroporated with Redd1 siRNA and were effective immune activators and drug carriers [104]. The absorption of paclitaxel by immunosuppressive macrophages was shown to enhance glycolysis [104], and the use of siRNA to repolarize tumor-associated macrophages (TAM) and increase tumor immune activation led to a shift in tumor metabolism and the subsequent suppression of tumor growth. In another application, “self-blockade” of tumor cell PD-L1 and improved treatment effectiveness in mice tumor models were achieved by the introduction of an encapsulated plasmid expressing PD-1 to cancer cells [130].
Besides loading therapeutics, several groups have developed engineered bEVs with targeting ligands to enhance specificity of delivery. Adriani et al targeted triple-negative breast cancer murine models using modified E. coli bEVs expressing an EGFR-targeting scFv version of panitumumab [105]. These scFv-bEVs bound to EGFR-overexpressed cancer cells more than control bEVs and also displayed enhanced tumor targeting in EGFR-expressing models in vivo [105]. Similar to prior studies, these bEVs re-polarizated macrophages to an inflammatory phenotype and potentiated the recruitment of cytotoxic CD8+ T lymphocytes [105]. Engineered E. coli strain W3110 bEVs with surface PD-1 ectodomain also demonstrated potent anti-tumor responses in vivo [106]. The modified PD-1-bEVs attach to PD-L1 on the surface of cancer cells, causing it to be internalized and decreasing surface expression, therefore shielding attracted T cells from immune suppressive signals of the PD-1/PD-L1 axis [106].
As technologies for loading and targeting are expanding, studies are beginning to combine the 2 approaches for further enhancement of therapeutic effects. For example, the E. coli K-12 W3110 strain was modified to present bEV-associated HER2-specific antibody on the surface by fusion to Cytolysin A and loaded with siRNA targeting kinesin spindle protein [107]. These modified bEVs exhibited cytotoxic effects on cell lines that overexpress HER2 (SKOV3, BT474, and HCC-1954) when tested in vitro. Additionally, in vivo experiments using HCC-1954 xenografts have shown that bEVs significantly suppressed tumor development compared to the control group treated with a vehicle. Others have tested the use of engineered bEVs in combination with synthetic nanomaterials to generate hybrid nanoformulations. Chen et al modified attenuated Salmonella bEVs to display RGD peptide by functionalization with DSPE-PEG-RGD for cellular targeting [108]. They then combined these bEVs with polymeric micelles encapsulating the 5-fluorouracil (5-FU) prodrug tegafur by extrusion. This hybrid nanoformulation further enhanced tumor cytotoxicity and suppressed tumor development and metastatic nodules in several preclinical mouse tumor models [108]. DSPE-PEG-RGD-based targeting was also employed on bEVs from the magnetotactic bacterium Magnetospirillum gryphiswaldense, cultured in the presence of iron and doxorubicin to generate bEVs loaded with all 3 of these components [109]. These bEVs resulted in ferroptosis of targeted cancer cells and immune activation and displayed preclinical therapeutic efficacy in primary and metastic breast cancer models [109]. Cui et al also generated a hybrid nanoformulation consisting of miRNA encapsulated in a zeolitic imidazolate framework-8 and then coated with E. coli bEVs expressing surface PD-1/Cytolysin A fusion protein as a tumor-targeting ligand [110]. These hybrid nanoparticles effectively delivered siRNA intravenously to mouse breast cancer models in vivo, significantly delaying tumor growth [110]. In another study, Mi et al employed a tandem bacteriotherapy/bEV strategy to target brain tumors, a long-standing challenge in drug development, given the blood-brain barrier [94]. Specifically, they showed that bacteriotherapy of brain tumors with attenuated Salmonella enhanced accumulation of Salmonella bEVs loaded with doxorubicin via a hitchhiking process on circulating neutrophils (ie, bEVs associated with neutrophils in circulation, which the authors claim helped bring them to tumors) [94]. Given the complexity of this strategy, it remains to be seen whether such approaches will find broader application.
Bacterial EVs in cancer vaccine research. Several bEV-based acellular vaccines have previously established the potential of bEVs to generate an adaptive memory immune response [131]. The most notable examples are vaccines against Neisseria meningitidis serogroup B, of which there are clinically used products [132, 133]. In addition, several studies have demonstrated the efficacy of bEVs derived from several gram-positive bacteria, such as Clostridium perfringens, Streptococcus pneumoniae, Bacillus anthracis, Mycobacterium tuberculosis, and Staphylococcus aureus, as vaccines against infections in mouse models [134]. These bEVs have been shown to significantly improve survival rates following exposure to a lethal challenge with corresponding bacteria in preclinical models [134].
In the last decade, bEVs have been proposed as an appealing candidate for cancer vaccines owing to their notable immunogenicity, lack of proliferative capacity, and ability to transport and display custom target antigens on their membranes [135, 136]. These bEV cancer vaccines typically involve genetic engineering to introduce compounds such as foreign proteins and small RNAs into the vesicle lumen or onto the membrane surface, where they can act as antigens to stimulate an immune response while preserving the vaccine’s natural immunogenicity and minimizing any potential adverse effects [135]. For instance, Grandi et al demonstrated that administration of E. coli bEVs, engineered to express an epitope of the tumor-enriched antigen FAT1, generated tumor-targeting antibodies that protected mice from CT26 murine colon adenocarcinoma [137]. The same group also developed E. coli bEVs expressing peptide epitopes of EGFR-EGFRvIII by fusion to the Neiserria meningitidis factor H binding-vIII protein (Nm-fHbp) and described a vaccine-induced antibody response that was associated with inhibition of EGFRvIII-expressing B16F10 tumor growth in mouse models [138]. Moreover, bEVs have also been used to develop auto-antibodies against tumor promoting growth factors, such as FGF, which also provided protection against tumor growth in preclinical models [139].
To facilitate facile coating of bEVs with diverse tumor antigens, Cheng et al developed bEVs expressing Cytolysin A fused to SpyCatcher and SnoopCatcher peptides, which form covalent bonds with antigens fused to SpyTag and SnoopTag peptides, respectively [140]. In their proof-of-concept investigations, the researchers demonstrated this methodology can induce T-cell reactions against several tumor antigens, resulting in a significant impairment in tumor growth in mouse models of lung cancer [140]. Similarly, Li et al developed engineered bEVs expressing the RNA binding protein L7Ae, which allowed for post-purification binding to select mRNA vaccine constructs for tumor antigens [141]. Plug-and-play approaches such as these could comprise useful platforms for the rapid generation of personalized vaccines using off-the-shelf bEVs and custom-synthesized peptide or mRNA pools.
In addition to custom immunogen loading, others have further modified bEVs to enhance their efficacy as vaccines or combination vaccine/therapeutics. They incorporated the lysosomal escape protein listeriolysin O to facilitate and enhance the expression of the delivered mRNAs after cellular uptake [141]. In another combination approach, Liang et al developed a trained-immunity-related vaccine using bEVs expressing SIRPα to facilitate phagocytosis by antigen-presenting cells and GM-CSF to further activate these cells [142]. They showed that this vaccine, produced using E. coli bEVs, reduced subcutaneous tumor development in 2 models: MC38 (TAM-rich with low in T cells) and B16-F10 (T cell-rich with low TAM). They also reported that MC38 antitumor mechanisms relied on both trained innate immunity and activated T-cell response, whereas B16-F10 responses predominantly relied on trained innate immunity, revealing that modulating macrophage activity within tumors is essential for vaccine-induced immunotherapy [142].
As in the case of immunotherapeutic bEVs, further understanding of bEV-immune interaction mechanisms will be instrumental in the further development of this platform as a form of novel cancer vaccines.
The understanding of the spectrum of bEV biological effects is facilitating the development of innovative therapeutic technologies; bEVs possess the capability to transfer biological cargo such as DNA, RNA, proteins, and lipids, providing at least partial protection against breakdown by the host organism [143]. The ability to tailor the interactions and functionalities of these biological nanoparticles suggests that bEVs may find use in cutting-edge diagnostic and therapeutic applications [96]. The anti-tumor effects demonstrated with the use of bEVs in preclinical cancer models (Figure 2) capitalize on a range of features, including inherent immunomodulatory capabilities, favorable potential for large-scale production, adaptability for personalized therapies, and enhanced safety profiles [144].
Despite these early promising results, there exist several significant hurdles in the transition of bEVs from bench-to-bedside clinical applications, some of which were summarized in position papers of the International Society for Extracellular Vesicles [145] and Chinese Society for Extracellular Vesicles [146]. These concerns include toxicity of bacterial components, consistency of purification, and cost of production.
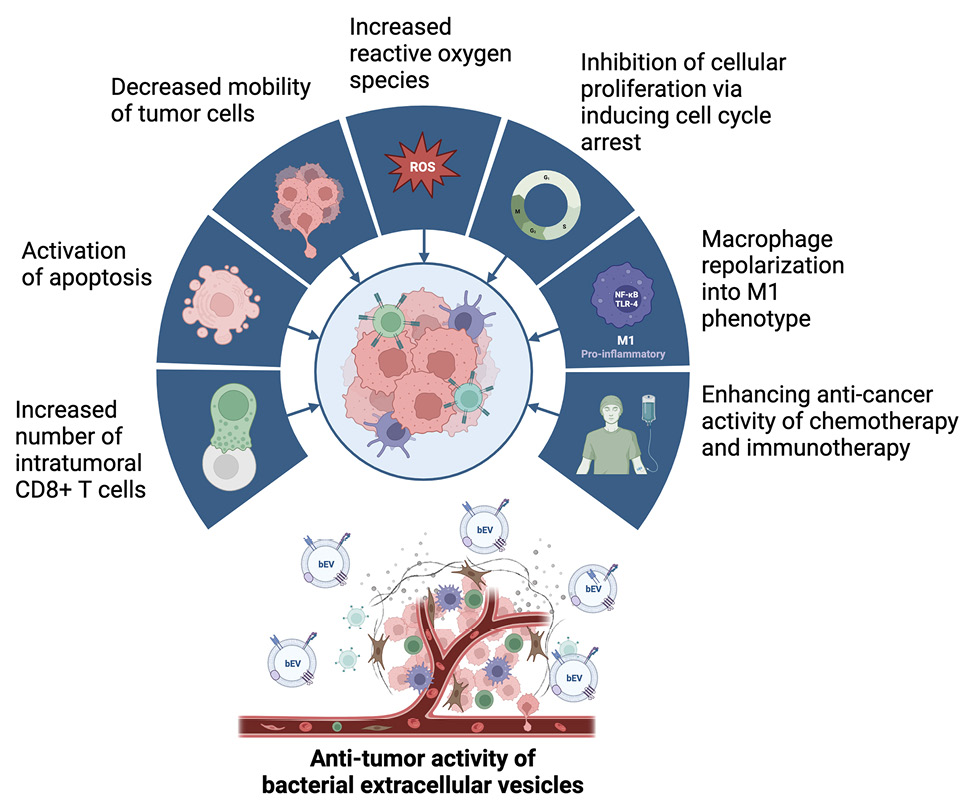
Figure 2. Proposed mechanisms of action of bacterial extracellular vesicles with anti-tumor effects observed in preclinical models. Created with BioRender.com.
Regulatory agencies generally classify bEV-based treatments as biologics [147]. Although bEVs exhibit reduced susceptibility to post-purification alterations in comparison to their progenitor cells, their analytical characterization and manufacturing processes pose more complexities when compared to other biologics often employed in clinical settings, such as monoclonal antibodies, in particular, due to their heterogeneity in size, origin, and composition. Indeed, regulatory clearance will likely require that bEVs meet certain purity standards during manufacturing in addition to showing efficacy and safety [148]. Given the breadth of possible bEV sources and engineering modifications, it is likely that some of these will have to be determined on a case-by-case basis. Still, one could envision certain common criteria (for example, endotoxin activity or lack of high-molecular-weight, non-vesicular component contamination, such as gram-negative bacterial fimbriae [149] or the Factor H binding protein from Neisseria) [150]. Some of these criteria may need to comprise part of even early preclinical development to avoid investment of resources in candidate therapeutic bEVs that do not have beneficial properties.
Despite any process standardization efforts, challenges with the concept of bEV purity will most likely remain. No current technologies exist that can produce “pure” EVs [151], while technologies that come closest (such as immunoisolation) [152] are not practically scalable. Moreover, discoveries such as that of a protein corona adsorbed to the surface of EVs with functional ramifications [153] raises the possibility that more purity may not always be beneficial. The biophysical overlap of putative contaminants with subsets of the heterogeneous EVs poses additional challenges, as methods that deplete one may affect the relative makeup of the other. In summary, perhaps more so than in the case of recombinant proteins, the industrial phrase “the process is the product” is highly applicable to the development of bEV therapeutics. In practice, dealing with this challenge may involve testing the impact of different bEV-enrichment strategies on desired functional attributes and toxicity of candidate products [145]. This approach goes together with a need for method standardization and transparency (for example, through EV-TRACK platform reporting) [154]. In addition, once methods are standardized for a given product, multiple orthogonal metrics of purity can be employed. These could include enrichment of total vesicular particles (measured by methods such as nanoparticle tracking analysis, tunable resistive pulse sensing, magnetic resistive pulse sensing, or nano-flow cytometry) [155]; particle-to-protein ratio [156]; enrichment of target marker proteins; and/or depletion of established undesired contaminants for a given bEV source. Finally, given the invariable presence of non-vesicular components in preparations, it is important to link function specifically to the bEVs themselves as opposed to the contaminants. Experimental approaches to this are still evolving and could include things like loss-of-function upon treatment with detergents, retaining function upon treatment with proteases/nucleases, or retaining function in small-scale preparations of the highest purity possible.
Regarding mass production of bEVs, a significant benefit of a bacterial source as opposed to developing therapeutic EVs from a mammalian cell source is the ease of scaling up the process (usually in the form of bioreactor cultures). Several studies have described ways of improving bEV yields in these settings by additional environmental stresses including high pressure, high temperature, nutritional deficiency, shear stress, and ethanol [157, 158]. It remains to be seen whether these strategies will retain the desired functionality of purified bEVs. Regarding downstream purification, most development involves employing tangential flow filtration, followed by size exclusion or affinity chromatography [149, 159]. Recently, Won et al piloted a modified large-scale manufacturing process by combining metal precipitation and size‐exclusion chromatography, which succeeded in cost-effectively mass-producing E. coli bEVs [99]. They compared the results of this novel technique to those obtained by using more traditional approaches with ultracentrifugation and buoyant density gradient ultracentrifugation [40, 160], observing yield and purity at least as good as those observed with other less scalable technologies. These studies comprise important first steps in developing workflows for efficient mass production of bEVs for further clinical development.
While past experience with bacteria-derived therapeutics and ongoing research are likely to overcome these technical challenges, there is still much to learn about the pharmacologic properties of bEVs. At a fundamental level, it is difficult to a priori predict the biological effects of bEVs, some of which can even have immunosuppressive properties [161]. In several published studies, the results of in vivo preclinical experiments are in line with the biological effects of bEVs modeled through in vitro functional assays [162, 163]. Applications of technologies used in the synthetic nanoparticle and mammalian EV fields can further improve the ability to predict and modulate the properties of bEVs. For instance, by modifying nanoparticles to incorporate fluorescent markers, researchers can see the gradual incorporation and movement of bEVs into immune cells or organ-on-chip models as they happen in real-time [164]. Magnetic nanoparticles can also change bEV’s spatial distribution, allowing researchers to study how their localization influences human-cell interaction [165]. Moreover, encapsulating bEVs in synthetic nanoparticles may improve their stability and bioavailability [166]. Combining these strategies may comprise promising solutions to optimize the pharmacologic properties of bEVs.
A vast majority of in vivo studies have utilized mouse models, and it remains unclear how other mammalian (especially primate) immune systems will respond to these complex agents. Given the established distinct characteristics of murine immune systems [167, 168], preclinical testing in non-human primates is likely to represent a critical step in the development of therapeutic bEVs for at least some applications. This is true both to elucidate on-target immune effects and to assess toxicity, especially as multiple groups are working on engineering “attenuated” bacteria that produce bEVs with presumed less systemic toxicity [108, 169, 170].
Another major concern is the development of anti-drug antibodies or other forms of anti-drug immune response against a repeatedly administered xenobiotic drug. Long-term, repetitive administration of non-human EVs can result in either immunogenicity or allergenicity [171, 172]. Qing et al attempted to prevent the formation of anti-drug antibodies by encapsulating E. coli bEVs in biocompatible calcium phosphate which dissolves in acidic TME to release the bEV. When paired with a photosensitizer, these encapsulated bEVs elicited photothermal immunogenic cell death in CT26 solid tumor mice and 4T1 tumor mice [173]. It remains to be seen whether engineering approaches such as these are necessary to retain therapeutic properties of bEVs administered repeatedly.
Studies investigating potential therapeutic applications of bEVs for cancer immunotherapy have been significantly increasing over the years, with promising results. However, if they are to be further developed as a therapeutic platform, our understanding of their biologic and pharmacologic properties in model systems and primates will need to significantly expand. The same is true for identifying bEV sources with desirable intrinsic properties and/or designing engineering modifications to improve the safety and efficacy of potential therapeutic bEVs. Elucidating mechanisms of biogenesis, heterogeneity, and downstream molecular signaling on tumor and microenvironment cells is critical for taking this technology to more advanced stages of drug development. Moreover, this will enable the rational combination of bEV therapeutics with other modalities, such as various immunotherapy approaches. Advancements in understanding these biological parameters, together with improvements and standardization of technologies to manufacture and characterize bEVs and their pharmacologic properties will help transform this field from proof-of-principle to actual clinical application as a versatile and promising therapeutic platform for cancer.
This work was supported by Sylvester Comprehensive Cancer Center Start-Up Funds (D.B.) and National Institutes of Health grants R00CA248611 (D.B.) and K99CA277242 (D.C.W.).
The authors have no conflicts of interest to declare.
1. Peroni DG, Nuzzi G, Trambusti I, Di Cicco ME, Comberiati P. Microbiome Composition and Its Impact on the Development of Allergic Diseases. Front Immunol. 2020;11:700. doi: 10.3389/fimmu.2020.00700. PubMed PMID: 32391012; PMCID: PMC7191078.
2. Dzutsev A, Goldszmid RS, Viaud S, Zitvogel L, Trinchieri G. The role of the microbiota in inflammation, carcinogenesis, and cancer therapy. Eur J Immunol. 2015;45(1):17-31. doi: 10.1002/eji.201444972. PubMed PMID: 25328099.
3. Chiller K, Selkin BA, Murakawa GJ. Skin microflora and bacterial infections of the skin. J Investig Dermatol Symp Proc. 2001;6(3):170-4. doi: 10.1046/j.0022-202x.2001.00043.x. PubMed PMID: 11924823.
4. Natalini JG, Singh S, Segal LN. The dynamic lung microbiome in health and disease. Nat Rev Microbiol. 2023;21(4):222-35. doi: 10.1038/s41579-022-00821-x. PubMed PMID: 36385637; PMCID: PMC9668228.
5. Perez-Carrasco V, Soriano-Lerma A, Soriano M, Gutierrez-Fernandez J, Garcia-Salcedo JA. Urinary Microbiome: Yin and Yang of the Urinary Tract. Front Cell Infect Microbiol. 2021;11:617002. doi: 10.3389/fcimb.2021.617002. PubMed PMID: 34084752; PMCID: PMC8167034.
6. Castillo DJ, Rifkin RF, Cowan DA, Potgieter M. The Healthy Human Blood Microbiome: Fact or Fiction? Front Cell Infect Microbiol. 2019;9:148. doi: 10.3389/fcimb.2019.00148. PubMed PMID: 31139578; PMCID: PMC6519389.
7. Rastelli M, Cani PD, Knauf C. The Gut Microbiome Influences Host Endocrine Functions. Endocr Rev. 2019;40(5):1271-84. doi: 10.1210/er.2018-00280. PubMed PMID: 31081896.
8. Rastelli M, Knauf C, Cani PD. Gut Microbes and Health: A Focus on the Mechanisms Linking Microbes, Obesity, and Related Disorders. Obesity (Silver Spring). 2018;26(5):792-800. doi: 10.1002/oby.22175. PubMed PMID: 29687645; PMCID: PMC5947576.
9. Masenga SK, Hamooya B, Hangoma J, Hayumbu V, Ertuglu LA, Ishimwe J, Rahman S, Saleem M, Laffer CL, Elijovich F, Kirabo A. Recent advances in modulation of cardiovascular diseases by the gut microbiota. J Hum Hypertens. 2022;36(11):952-9. doi: 10.1038/s41371-022-00698-6. PubMed PMID: 35469059; PMCID: PMC9649420.
10. Graham DB, Xavier RJ. Conditioning of the immune system by the microbiome. Trends Immunol. 2023;44(7):499-511. doi: 10.1016/j.it.2023.05.002. PubMed PMID: 37236891.
11. Kaparakis-Liaskos M, Ferrero RL. Immune modulation by bacterial outer membrane vesicles. Nat Rev Immunol. 2015;15(6):375-87. doi: 10.1038/nri3837. PubMed PMID: 25976515.
12. Zimmermann M, Zimmermann-Kogadeeva M, Wegmann R, Goodman AL. Mapping human microbiome drug metabolism by gut bacteria and their genes. Nature. 2019;570(7762):462-7. doi: 10.1038/s41586-019-1291-3. PubMed PMID: 31158845; PMCID: PMC6597290.
13. Helmink BA, Khan MAW, Hermann A, Gopalakrishnan V, Wargo JA. The microbiome, cancer, and cancer therapy. Nat Med. 2019;25(3):377-88. doi: 10.1038/s41591-019-0377-7. PubMed PMID: 30842679.
14. Schwabe RF, Jobin C. The microbiome and cancer. Nat Rev Cancer. 2013;13(11):800-12. doi: 10.1038/nrc3610. PubMed PMID: 24132111; PMCID: PMC3986062.
15. Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, Molina DA, Salcedo R, Back T, Cramer S, Dai RM, Kiu H, Cardone M, Naik S, Patri AK, Wang E, Marincola FM, Frank KM, Belkaid Y, Trinchieri G, Goldszmid RS. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342(6161):967-70. doi: 10.1126/science.1240527. PubMed PMID: 24264989; PMCID: PMC6709532.
16. Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman K, Wei SC, Cogdill AP, Zhao L, Hudgens CW, Hutchinson DS, Manzo T, Petaccia de Macedo M, Cotechini T, Kumar T, Chen WS, Reddy SM, Szczepaniak Sloane R, Galloway-Pena J, Jiang H, Chen PL, Shpall EJ, Rezvani K, Alousi AM, Chemaly RF, Shelburne S, Vence LM, Okhuysen PC, Jensen VB, Swennes AG, McAllister F, Marcelo Riquelme Sanchez E, Zhang Y, Le Chatelier E, Zitvogel L, Pons N, Austin-Breneman JL, Haydu LE, Burton EM, Gardner JM, Sirmans E, Hu J, Lazar AJ, Tsujikawa T, Diab A, Tawbi H, Glitza IC, Hwu WJ, Patel SP, Woodman SE, Amaria RN, Davies MA, Gershenwald JE, Hwu P, Lee JE, Zhang J, Coussens LM, Cooper ZA, Futreal PA, Daniel CR, Ajami NJ, Petrosino JF, Tetzlaff MT, Sharma P, Allison JP, Jenq RR, Wargo JA. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359(6371):97-103. doi: 10.1126/science.aan4236. PubMed PMID: 29097493; PMCID: PMC5827966.
17. Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre ML, Luke JJ, Gajewski TF. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018;359(6371):104-8. doi: 10.1126/science.aao3290. PubMed PMID: 29302014; PMCID: PMC6707353.
18. Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillere R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP, Fidelle M, Flament C, Poirier-Colame V, Opolon P, Klein C, Iribarren K, Mondragon L, Jacquelot N, Qu B, Ferrere G, Clemenson C, Mezquita L, Masip JR, Naltet C, Brosseau S, Kaderbhai C, Richard C, Rizvi H, Levenez F, Galleron N, Quinquis B, Pons N, Ryffel B, Minard-Colin V, Gonin P, Soria JC, Deutsch E, Loriot Y, Ghiringhelli F, Zalcman G, Goldwasser F, Escudier B, Hellmann MD, Eggermont A, Raoult D, Albiges L, Kroemer G, Zitvogel L. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359(6371):91-7. doi: 10.1126/science.aan3706. PubMed PMID: 29097494.
19. Davar D, Dzutsev AK, McCulloch JA, Rodrigues RR, Chauvin JM, Morrison RM, Deblasio RN, Menna C, Ding Q, Pagliano O, Zidi B, Zhang S, Badger JH, Vetizou M, Cole AM, Fernandes MR, Prescott S, Costa RGF, Balaji AK, Morgun A, Vujkovic-Cvijin I, Wang H, Borhani AA, Schwartz MB, Dubner HM, Ernst SJ, Rose A, Najjar YG, Belkaid Y, Kirkwood JM, Trinchieri G, Zarour HM. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science. 2021;371(6529):595-602. doi: 10.1126/science.abf3363. PubMed PMID: 33542131; PMCID: PMC8097968.
20. Routy B, Lenehan JG, Miller WH, Jr., Jamal R, Messaoudene M, Daisley BA, Hes C, Al KF, Martinez-Gili L, Puncochar M, Ernst S, Logan D, Belanger K, Esfahani K, Richard C, Ninkov M, Piccinno G, Armanini F, Pinto F, Krishnamoorthy M, Figueredo R, Thebault P, Takis P, Magrill J, Ramsay L, Derosa L, Marchesi JR, Parvathy SN, Elkrief A, Watson IR, Lapointe R, Segata N, Haeryfar SMM, Mullish BH, Silverman MS, Burton JP, Maleki Vareki S. Fecal microbiota transplantation plus anti-PD-1 immunotherapy in advanced melanoma: a phase I trial. Nat Med. 2023;29(8):2121-32. doi: 10.1038/s41591-023-02453-x. PubMed PMID: 37414899.
21. Derosa L, Routy B, Thomas AM, Iebba V, Zalcman G, Friard S, Mazieres J, Audigier-Valette C, Moro-Sibilot D, Goldwasser F, Silva CAC, Terrisse S, Bonvalet M, Scherpereel A, Pegliasco H, Richard C, Ghiringhelli F, Elkrief A, Desilets A, Blanc-Durand F, Cumbo F, Blanco A, Boidot R, Chevrier S, Daillere R, Kroemer G, Alla L, Pons N, Le Chatelier E, Galleron N, Roume H, Dubuisson A, Bouchard N, Messaoudene M, Drubay D, Deutsch E, Barlesi F, Planchard D, Segata N, Martinez S, Zitvogel L, Soria JC, Besse B. Intestinal Akkermansia muciniphila predicts clinical response to PD-1 blockade in patients with advanced non-small-cell lung cancer. Nat Med. 2022;28(2):315-24. doi: 10.1038/s41591-021-01655-5. PubMed PMID: 35115705; PMCID: PMC9330544.
22. Lee KA, Thomas AM, Bolte LA, Bjork JR, de Ruijter LK, Armanini F, Asnicar F, Blanco-Miguez A, Board R, Calbet-Llopart N, Derosa L, Dhomen N, Brooks K, Harland M, Harries M, Leeming ER, Lorigan P, Manghi P, Marais R, Newton-Bishop J, Nezi L, Pinto F, Potrony M, Puig S, Serra-Bellver P, Shaw HM, Tamburini S, Valpione S, Vijay A, Waldron L, Zitvogel L, Zolfo M, de Vries EGE, Nathan P, Fehrmann RSN, Bataille V, Hospers GAP, Spector TD, Weersma RK, Segata N. Cross-cohort gut microbiome associations with immune checkpoint inhibitor response in advanced melanoma. Nat Med. 2022;28(3):535-44. doi: 10.1038/s41591-022-01695-5. PubMed PMID: 35228751; PMCID: PMC8938272.
23. Doki N, Suyama M, Sasajima S, Ota J, Igarashi A, Mimura I, Morita H, Fujioka Y, Sugiyama D, Nishikawa H, Shimazu Y, Suda W, Takeshita K, Atarashi K, Hattori M, Sato E, Watakabe-Inamoto K, Yoshioka K, Najima Y, Kobayashi T, Kakihana K, Takahashi N, Sakamaki H, Honda K, Ohashi K. Clinical impact of pre-transplant gut microbial diversity on outcomes of allogeneic hematopoietic stem cell transplantation. Ann Hematol. 2017;96(9):1517-23. doi: 10.1007/s00277-017-3069-8. PubMed PMID: 28733895.
24. Calvo-Barreiro L, Zhang L, Abdel-Rahman SA, Naik SP, Gabr M. Gut Microbial-Derived Metabolites as Immune Modulators of T Helper 17 and Regulatory T Cells. Int J Mol Sci. 2023;24(2). doi: 10.3390/ijms24021806. PubMed PMID: 36675320; PMCID: PMC9867388.
25. Mao YQ, Huang JT, Zhang SL, Kong C, Li ZM, Jing H, Chen HL, Kong CY, Huang SH, Cai PR, Han B, Wang LS. The antitumour effects of caloric restriction are mediated by the gut microbiome. Nat Metab. 2023;5(1):96-110. doi: 10.1038/s42255-022-00716-4. PubMed PMID: 36646754.
26. Masse KE, Lu VB. Short-chain fatty acids, secondary bile acids and indoles: gut microbial metabolites with effects on enteroendocrine cell function and their potential as therapies for metabolic disease. Front Endocrinol (Lausanne). 2023;14:1169624. doi: 10.3389/fendo.2023.1169624. PubMed PMID: 37560311; PMCID: PMC10407565.
27. Nejman D, Livyatan I, Fuks G, Gavert N, Zwang Y, Geller LT, Rotter-Maskowitz A, Weiser R, Mallel G, Gigi E, Meltser A, Douglas GM, Kamer I, Gopalakrishnan V, Dadosh T, Levin-Zaidman S, Avnet S, Atlan T, Cooper ZA, Arora R, Cogdill AP, Khan MAW, Ologun G, Bussi Y, Weinberger A, Lotan-Pompan M, Golani O, Perry G, Rokah M, Bahar-Shany K, Rozeman EA, Blank CU, Ronai A, Shaoul R, Amit A, Dorfman T, Kremer R, Cohen ZR, Harnof S, Siegal T, Yehuda-Shnaidman E, Gal-Yam EN, Shapira H, Baldini N, Langille MGI, Ben-Nun A, Kaufman B, Nissan A, Golan T, Dadiani M, Levanon K, Bar J, Yust-Katz S, Barshack I, Peeper DS, Raz DJ, Segal E, Wargo JA, Sandbank J, Shental N, Straussman R. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science. 2020;368(6494):973-80. doi: 10.1126/science.aay9189. PubMed PMID: 32467386; PMCID: PMC7757858.
28. Silva-Valenzuela CA, Desai PT, Molina-Quiroz RC, Pezoa D, Zhang Y, Porwollik S, Zhao M, Hoffman RM, Contreras I, Santiviago CA, McClelland M. Solid tumors provide niche-specific conditions that lead to preferential growth of Salmonella. Oncotarget. 2016;7(23):35169-80. doi: 10.18632/oncotarget.9071. PubMed PMID: 27145267; PMCID: PMC5085218.
29. Riquelme E, Zhang Y, Zhang L, Montiel M, Zoltan M, Dong W, Quesada P, Sahin I, Chandra V, San Lucas A, Scheet P, Xu H, Hanash SM, Feng L, Burks JK, Do KA, Peterson CB, Nejman D, Tzeng CD, Kim MP, Sears CL, Ajami N, Petrosino J, Wood LD, Maitra A, Straussman R, Katz M, White JR, Jenq R, Wargo J, McAllister F. Tumor Microbiome Diversity and Composition Influence Pancreatic Cancer Outcomes. Cell. 2019;178(4):795-806 e12. doi: 10.1016/j.cell.2019.07.008. PubMed PMID: 31398337; PMCID: PMC7288240.
30. Gihawi A, Ge Y, Lu J, Puiu D, Xu A, Cooper CS, Brewer DS, Pertea M, Salzberg SL. Major data analysis errors invalidate cancer microbiome findings. bioRxiv. 2023. doi: 10.1101/2023.07.28.550993. PubMed PMID: 37577699; PMCID: PMC10418105.
31. Poore GD, Kopylova E, Zhu Q, Carpenter C, Fraraccio S, Wandro S, Kosciolek T, Janssen S, Metcalf J, Song SJ, Kanbar J, Miller-Montgomery S, Heaton R, McKay R, Patel SP, Swafford AD, Knight R. Microbiome analyses of blood and tissues suggest cancer diagnostic approach. Nature. 2020;579(7800):567-74. doi: 10.1038/s41586-020-2095-1. PubMed PMID: 32214244; PMCID: PMC7500457.
32. Toyofuku M, Schild S, Kaparakis-Liaskos M, Eberl L. Composition and functions of bacterial membrane vesicles. Nat Rev Microbiol. 2023;21(7):415-30. doi: 10.1038/s41579-023-00875-5. PubMed PMID: 36932221.
33. Nahui Palomino RA, Vanpouille C, Costantini PE, Margolis L. Microbiota-host communications: Bacterial extracellular vesicles as a common language. PLoS Pathog. 2021;17(5):e1009508. doi: 10.1371/journal.ppat.1009508. PubMed PMID: 33984071; PMCID: PMC8118305.
34. Xie J, Haesebrouck F, Van Hoecke L, Vandenbroucke RE. Bacterial extracellular vesicles: an emerging avenue to tackle diseases. Trends Microbiol. 2023;31(12):1206-24. doi: 10.1016/j.tim.2023.05.010. PubMed PMID: 37330381.
35. Kurata A, Kiyohara S, Imai T, Yamasaki-Yashiki S, Zaima N, Moriyama T, Kishimoto N, Uegaki K. Characterization of extracellular vesicles from Lactiplantibacillus plantarum. Sci Rep. 2022;12(1):13330. doi: 10.1038/s41598-022-17629-7. PubMed PMID: 35941134; PMCID: PMC9360025.
36. Shen Y, Giardino Torchia ML, Lawson GW, Karp CL, Ashwell JD, Mazmanian SK. Outer membrane vesicles of a human commensal mediate immune regulation and disease protection. Cell Host Microbe. 2012;12(4):509-20. doi: 10.1016/j.chom.2012.08.004. PubMed PMID: 22999859; PMCID: PMC3895402.
37. Shin TS, Park JY, Kim YK, Kim JG. Extracellular vesicles derived from small intestinal lamina propria reduce antigen-specific immune response. Korean J Intern Med. 2022;37(1):85-95. doi: 10.3904/kjim.2020.510. PubMed PMID: 34425655; PMCID: PMC8747917.
38. Chronopoulos A, Kalluri R. Emerging role of bacterial extracellular vesicles in cancer. Oncogene. 2020;39(46):6951-60. doi: 10.1038/s41388-020-01509-3. PubMed PMID: 33060855; PMCID: PMC7557313.
39. Han L, Lam EW, Sun Y. Extracellular vesicles in the tumor microenvironment: old stories, but new tales. Mol Cancer. 2019;18(1):59. doi: 10.1186/s12943-019-0980-8. PubMed PMID: 30925927; PMCID: PMC6441234.
40. Kim OY, Park HT, Dinh NTH, Choi SJ, Lee J, Kim JH, Lee SW, Gho YS. Bacterial outer membrane vesicles suppress tumor by interferon-gamma-mediated antitumor response. Nat Commun. 2017;8(1):626. doi: 10.1038/s41467-017-00729-8. PubMed PMID: 28931823; PMCID: PMC5606984.
41. Macia L, Nanan R, Hosseini-Beheshti E, Grau GE. Host- and Microbiota-Derived Extracellular Vesicles, Immune Function, and Disease Development. Int J Mol Sci. 2019;21(1). doi: 10.3390/ijms21010107. PubMed PMID: 31877909; PMCID: PMC6982009.
42. Craven DE, Peppler MS, Frasch CE, Mocca LF, McGrath PP, Washington G. Adherence of isolates of Neisseria meningitidis from patients and carriers to human buccal epithelial cells. J Infect Dis. 1980;142(4):556-68. doi: 10.1093/infdis/142.4.556. PubMed PMID: 6108344.
43. DeVoe IW, Gilchrist JE. Pili on meningococci from primary cultures of nasopharyngeal carriers and cerebrospinal fluid of patients with acute disease. J Exp Med. 1975;141(2):297-305. doi: 10.1084/jem.141.2.297. PubMed PMID: 803544; PMCID: PMC2190534.
44. Fiocca R, Necchi V, Sommi P, Ricci V, Telford J, Cover TL, Solcia E. Release of Helicobacter pylori vacuolating cytotoxin by both a specific secretion pathway and budding of outer membrane vesicles. Uptake of released toxin and vesicles by gastric epithelium. J Pathol. 1999;188(2):220-6. doi: 10.1002/(SICI)1096-9896(199906)188:2<220::AID-PATH307>3.0.CO;2-C. PubMed PMID: 10398168.
45. Keenan J, Day T, Neal S, Cook B, Perez-Perez G, Allardyce R, Bagshaw P. A role for the bacterial outer membrane in the pathogenesis of Helicobacter pylori infection. FEMS Microbiol Lett. 2000;182(2):259-64. doi: 10.1111/j.1574-6968.2000.tb08905.x. PubMed PMID: 10620676.
46. Hosseini-Giv N, Basas A, Hicks C, El-Omar E, El-Assaad F, Hosseini-Beheshti E. Bacterial extracellular vesicles and their novel therapeutic applications in health and cancer. Front Cell Infect Microbiol. 2022;12:962216. doi: 10.3389/fcimb.2022.962216. PubMed PMID: 36439225; PMCID: PMC9691856.
47. Costa Verdera H, Gitz-Francois JJ, Schiffelers RM, Vader P. Cellular uptake of extracellular vesicles is mediated by clathrin-independent endocytosis and macropinocytosis. J Control Release. 2017;266:100-8. doi: 10.1016/j.jconrel.2017.09.019. PubMed PMID: 28919558.
48. Jahromi LP, Fuhrmann G. Bacterial extracellular vesicles: Understanding biology promotes applications as nanopharmaceuticals. Adv Drug Deliv Rev. 2021;173:125-40. doi: 10.1016/j.addr.2021.03.012. PubMed PMID: 33774113.
49. O’Donoghue EJ, Sirisaengtaksin N, Browning DF, Bielska E, Hadis M, Fernandez-Trillo F, Alderwick L, Jabbari S, Krachler AM. Lipopolysaccharide structure impacts the entry kinetics of bacterial outer membrane vesicles into host cells. PLoS Pathog. 2017;13(11):e1006760. doi: 10.1371/journal.ppat.1006760. PubMed PMID: 29186191; PMCID: PMC5724897.
50. Rompikuntal PK, Thay B, Khan MK, Alanko J, Penttinen AM, Asikainen S, Wai SN, Oscarsson J. Perinuclear localization of internalized outer membrane vesicles carrying active cytolethal distending toxin from Aggregatibacter actinomycetemcomitans. Infect Immun. 2012;80(1):31-42. doi: 10.1128/IAI.06069-11. PubMed PMID: 22025516; PMCID: PMC3255663.
51. Jeong D, Kim MJ, Park Y, Chung J, Kweon HS, Kang NG, Hwang SJ, Youn SH, Hwang BK, Kim D. Visualizing extracellular vesicle biogenesis in gram-positive bacteria using super-resolution microscopy. BMC Biol. 2022;20(1):270. doi: 10.1186/s12915-022-01472-3. PubMed PMID: 36464676; PMCID: PMC9720944.
52. Mandelbaum N, Zhang L, Carasso S, Ziv T, Lifshiz-Simon S, Davidovich I, Luz I, Berinstein E, Gefen T, Cooks T, Talmon Y, Balskus EP, Geva-Zatorsky N. Extracellular vesicles of the Gram-positive gut symbiont Bifidobacterium longum induce immune-modulatory, anti-inflammatory effects. NPJ Biofilms Microbiomes. 2023;9(1):30. doi: 10.1038/s41522-023-00400-9. PubMed PMID: 37270554; PMCID: PMC10239484.
53. Wang X, Thompson CD, Weidenmaier C, Lee JC. Release of Staphylococcus aureus extracellular vesicles and their application as a vaccine platform. Nat Commun. 2018;9(1):1379. doi: 10.1038/s41467-018-03847-z. PubMed PMID: 29643357; PMCID: PMC5895597.
54. Altindis E, Fu Y, Mekalanos JJ. Proteomic analysis of Vibrio cholerae outer membrane vesicles. Proc Natl Acad Sci U S A. 2014;111(15):E1548-56. doi: 10.1073/pnas.1403683111. PubMed PMID: 24706774; PMCID: PMC3992640.
55. Pin C, David L, Oswald E. Modulation of Autophagy and Cell Death by Bacterial Outer-Membrane Vesicles. Toxins (Basel). 2023;15(8). doi: 10.3390/toxins15080502. PubMed PMID: 37624259; PMCID: PMC10467092.
56. Kawano K, Kamasaka K, Yokoyama F, Kawamoto J, Ogawa T, Kurihara T, Matsuzaki K. Structural factors governing binding of curvature-sensing peptides to bacterial extracellular vesicles covered with hydrophilic polysaccharide chains. Biophys Chem. 2023;299:107039. doi: 10.1016/j.bpc.2023.107039. PubMed PMID: 37209609.
57. Bitto NJ, Chapman R, Pidot S, Costin A, Lo C, Choi J, D’Cruze T, Reynolds EC, Dashper SG, Turnbull L, Whitchurch CB, Stinear TP, Stacey KJ, Ferrero RL. Bacterial membrane vesicles transport their DNA cargo into host cells. Sci Rep. 2017;7(1):7072. doi: 10.1038/s41598-017-07288-4. PubMed PMID: 28765539; PMCID: PMC5539193.
58. Kameli N, Borman R, Lpez-Iglesias C, Savelkoul P, Stassen FRM. Characterization of Feces-Derived Bacterial Membrane Vesicles and the Impact of Their Origin on the Inflammatory Response. Front Cell Infect Microbiol. 2021;11:667987. doi: 10.3389/fcimb.2021.667987. PubMed PMID: 34026664; PMCID: PMC8139245.
59. Stathatos I, Koumandou VL. Comparative Analysis of Prokaryotic Extracellular Vesicle Proteins and Their Targeting Signals. Microorganisms. 2023;11(8). doi: 10.3390/microorganisms11081977. PubMed PMID: 37630535; PMCID: PMC10458587.
60. Toyofuku M, Nomura N, Eberl L. Types and origins of bacterial membrane vesicles. Nat Rev Microbiol. 2019;17(1):13-24. doi: 10.1038/s41579-018-0112-2. PubMed PMID: 30397270.
61. Martin-Gallausiaux C, Malabirade A, Habier J, Wilmes P. Fusobacterium nucleatum Extracellular Vesicles Modulate Gut Epithelial Cell Innate Immunity via FomA and TLR2. Front Immunol. 2020;11:583644. doi: 10.3389/fimmu.2020.583644. PubMed PMID: 33408714; PMCID: PMC7779620.
62. van Bergenhenegouwen J, Kraneveld AD, Rutten L, Kettelarij N, Garssen J, Vos AP. Extracellular vesicles modulate host-microbe responses by altering TLR2 activity and phagocytosis. PLoS One. 2014;9(2):e89121. doi: 10.1371/journal.pone.0089121. PubMed PMID: 24586537; PMCID: PMC3930685.
63. Bitto NJ, Baker PJ, Dowling JK, Wray-McCann G, De Paoli A, Tran LS, Leung PL, Stacey KJ, Mansell A, Masters SL, Ferrero RL. Membrane vesicles from Pseudomonas aeruginosa activate the noncanonical inflammasome through caspase-5 in human monocytes. Immunol Cell Biol. 2018;96(10):1120-30. doi: 10.1111/imcb.12190. PubMed PMID: 30003588.
64. Thay B, Damm A, Kufer TA, Wai SN, Oscarsson J. Aggregatibacter actinomycetemcomitans outer membrane vesicles are internalized in human host cells and trigger NOD1- and NOD2-dependent NF-kappaB activation. Infect Immun. 2014;82(10):4034-46. doi: 10.1128/IAI.01980-14. PubMed PMID: 25024364; PMCID: PMC4187862.
65. Sivanantham A, Alktaish W, Murugeasan S, Gong B, Lee H, Jin Y. Caveolin-1 regulates OMV-induced macrophage pro-inflammatory activation and multiple Toll-like receptors. Front Immunol. 2023;14:1044834. doi: 10.3389/fimmu.2023.1044834. PubMed PMID: 36817491; PMCID: PMC9933776.
66. Singh PP, LeMaire C, Tan JC, Zeng E, Schorey JS. Exosomes released from M. tuberculosis infected cells can suppress IFN-gamma mediated activation of naive macrophages. PLoS One. 2011;6(4):e18564. doi: 10.1371/journal.pone.0018564. PubMed PMID: 21533172; PMCID: PMC3077381.
67. Kondo Y, Ito D, Taniguchi R, Tademoto S, Horie T, Otsuki H. Extracellular vesicles derived from Spirometra erinaceieuropaei plerocercoids inhibit activation of murine macrophage RAW264.7 cells. Parasitol Int. 2023;95:102742. doi: 10.1016/j.parint.2023.102742. PubMed PMID: 36870444.
68. Lee H, Zhang D, Laskin DL, Jin Y. Functional Evidence of Pulmonary Extracellular Vesicles in Infectious and Noninfectious Lung Inflammation. J Immunol. 2018;201(5):1500-9. doi: 10.4049/jimmunol.1800264. PubMed PMID: 29997122; PMCID: PMC6109965.
69. Machado RS, de Sousa IP, Jr., Monteiro JC, Ferreira JL, Dos Santos Alves JC, Tavares FN. Detection and identification of enteroviruses circulating in children with acute gastroenteritis in Para State, Northern Brazil (2010-2011). Virol J. 2020;17(1):156. doi: 10.1186/s12985-020-01431-w. PubMed PMID: 33066782; PMCID: PMC7565352.
70. Cha S, Seo EH, Lee SH, Kim KS, Oh CS, Moon JS, Kim JK. MicroRNA Expression in Extracellular Vesicles from Nasal Lavage Fluid in Chronic Rhinosinusitis. Biomedicines. 2021;9(5). doi: 10.3390/biomedicines9050471. PubMed PMID: 33925835; PMCID: PMC8145239.
71. Tiku V, Kofoed EM, Yan D, Kang J, Xu M, Reichelt M, Dikic I, Tan MW. Outer membrane vesicles containing OmpA induce mitochondrial fragmentation to promote pathogenesis of Acinetobacter baumannii. Sci Rep. 2021;11(1):618. doi: 10.1038/s41598-020-79966-9. PubMed PMID: 33436835; PMCID: PMC7804284.
72. Kang CS, Ban M, Choi EJ, Moon HG, Jeon JS, Kim DK, Park SK, Jeon SG, Roh TY, Myung SJ, Gho YS, Kim JG, Kim YK. Extracellular vesicles derived from gut microbiota, especially Akkermansia muciniphila, protect the progression of dextran sulfate sodium-induced colitis. PLoS One. 2013;8(10):e76520. doi: 10.1371/journal.pone.0076520. PubMed PMID: 24204633; PMCID: PMC3811976.
73. Chang X, Wang SL, Zhao SB, Shi YH, Pan P, Gu L, Yao J, Li ZS, Bai Y. Extracellular Vesicles with Possible Roles in Gut Intestinal Tract Homeostasis and IBD. Mediators Inflamm. 2020;2020:1945832. doi: 10.1155/2020/1945832. PubMed PMID: 32410847; PMCID: PMC7201673.
74. Koeppen K, Hampton TH, Jarek M, Scharfe M, Gerber SA, Mielcarz DW, Demers EG, Dolben EL, Hammond JH, Hogan DA, Stanton BA. A Novel Mechanism of Host-Pathogen Interaction through sRNA in Bacterial Outer Membrane Vesicles. PLoS Pathog. 2016;12(6):e1005672. doi: 10.1371/journal.ppat.1005672. PubMed PMID: 27295279; PMCID: PMC4905634.
75. Choi Y, Park HS, Kim YK. Bacterial Extracellular Vesicles: A Candidate Molecule for the Diagnosis and Treatment of Allergic Diseases. Allergy Asthma Immunol Res. 2023;15(3):279-89. doi: 10.4168/aair.2023.15.3.279. PubMed PMID: 37188485; PMCID: PMC10186123.
76. Lee JH, Choi JP, Yang J, Won HK, Park CS, Song WJ, Kwon HS, Kim TB, Kim YK, Park HS, Cho YS. Metagenome analysis using serum extracellular vesicles identified distinct microbiota in asthmatics. Sci Rep. 2020;10(1):15125. doi: 10.1038/s41598-020-72242-w. PubMed PMID: 32934287; PMCID: PMC7492258.
77. Lee DH, Park HK, Lee HR, Sohn H, Sim S, Park HJ, Shin YS, Kim YK, Choi Y, Park HS. Immunoregulatory effects of Lactococcus lactis-derived extracellular vesicles in allergic asthma. Clin Transl Allergy. 2022;12(3):e12138. doi: 10.1002/clt2.12138. PubMed PMID: 35344296; PMCID: PMC8967260.
78. Kim MH, Choi SJ, Choi HI, Choi JP, Park HK, Kim EK, Kim MJ, Moon BS, Min TK, Rho M, Cho YJ, Yang S, Kim YK, Kim YY, Pyun BY. Lactobacillus plantarum-derived Extracellular Vesicles Protect Atopic Dermatitis Induced by Staphylococcus aureus-derived Extracellular Vesicles. Allergy Asthma Immunol Res. 2018;10(5):516-32. doi: 10.4168/aair.2018.10.5.516. PubMed PMID: 30088371; PMCID: PMC6082821.
79. Kim JH, Jeun EJ, Hong CP, Kim SH, Jang MS, Lee EJ, Moon SJ, Yun CH, Im SH, Jeong SG, Park BY, Kim KT, Seoh JY, Kim YK, Oh SJ, Ham JS, Yang BG, Jang MH. Extracellular vesicle-derived protein from Bifidobacterium longum alleviates food allergy through mast cell suppression. J Allergy Clin Immunol. 2016;137(2):507-16 e8. doi: 10.1016/j.jaci.2015.08.016. PubMed PMID: 26433560.
80. Pilard C, Ancion M, Delvenne P, Jerusalem G, Hubert P, Herfs M. Cancer immunotherapy: it’s time to better predict patients’ response. Br J Cancer. 2021;125(7):927-38. doi: 10.1038/s41416-021-01413-x. PubMed PMID: 34112949; PMCID: PMC8476530.
81. Liu YT, Sun ZJ. Turning cold tumors into hot tumors by improving T-cell infiltration. Theranostics. 2021;11(11):5365-86. doi: 10.7150/thno.58390. PubMed PMID: 33859752; PMCID: PMC8039952.
82. Jorgovanovic D, Song M, Wang L, Zhang Y. Roles of IFN-gamma in tumor progression and regression: a review. Biomark Res. 2020;8:49. doi: 10.1186/s40364-020-00228-x. PubMed PMID: 33005420; PMCID: PMC7526126.
83. Sawant SS, Patil SM, Gupta V, Kunda NK. Microbes as Medicines: Harnessing the Power of Bacteria in Advancing Cancer Treatment. Int J Mol Sci. 2020;21(20). doi: 10.3390/ijms21207575. PubMed PMID: 33066447; PMCID: PMC7589870.
84. Sedighi M, Zahedi Bialvaei A, Hamblin MR, Ohadi E, Asadi A, Halajzadeh M, Lohrasbi V, Mohammadzadeh N, Amiriani T, Krutova M, Amini A, Kouhsari E. Therapeutic bacteria to combat cancer; current advances, challenges, and opportunities. Cancer Med. 2019;8(6):3167-81. doi: 10.1002/cam4.2148. PubMed PMID: 30950210; PMCID: PMC6558487.
85. Mills H, Acquah R, Tang N, Cheung L, Klenk S, Glassen R, Pirson M, Albert A, Hoang DT, Van TN. The Use of Bacteria in Cancer Treatment: A Review from the Perspective of Cellular Microbiology. Emerg Med Int. 2022;2022:8127137. doi: 10.1155/2022/8127137. PubMed PMID: 35978704; PMCID: PMC9377996.
86. Nallar SC, Xu DQ, Kalvakolanu DV. Bacteria and genetically modified bacteria as cancer therapeutics: Current advances and challenges. Cytokine. 2017;89:160-72. doi: 10.1016/j.cyto.2016.01.002. PubMed PMID: 26778055.
87. Song S, Vuai MS, Zhong M. The role of bacteria in cancer therapy - enemies in the past, but allies at present. Infect Agent Cancer. 2018;13:9. doi: 10.1186/s13027-018-0180-y. PubMed PMID: 29568324; PMCID: PMC5856380.
88. Jiang SN, Phan TX, Nam TK, Nguyen VH, Kim HS, Bom HS, Choy HE, Hong Y, Min JJ. Inhibition of tumor growth and metastasis by a combination of Escherichia coli-mediated cytolytic therapy and radiotherapy. Mol Ther. 2010;18(3):635-42. doi: 10.1038/mt.2009.295. PubMed PMID: 20051939; PMCID: PMC2839435.
89. Ryan RM, Green J, Williams PJ, Tazzyman S, Hunt S, Harmey JH, Kehoe SC, Lewis CE. Bacterial delivery of a novel cytolysin to hypoxic areas of solid tumors. Gene Ther. 2009;16(3):329-39. doi: 10.1038/gt.2008.188. PubMed PMID: 19177133.
90. McMillan HM, Kuehn MJ. Proteomic Profiling Reveals Distinct Bacterial Extracellular Vesicle Subpopulations with Possibly Unique Functionality. Appl Environ Microbiol. 2023;89(1):e0168622. doi: 10.1128/aem.01686-22. PubMed PMID: 36533919; PMCID: PMC9888257.
91. Biagiotti S, Abbas F, Montanari M, Barattini C, Rossi L, Magnani M, Papa S, Canonico B. Extracellular Vesicles as New Players in Drug Delivery: A Focus on Red Blood Cells-Derived EVs. Pharmaceutics. 2023;15(2). doi: 10.3390/pharmaceutics15020365. PubMed PMID: 36839687; PMCID: PMC9961903.
92. Marzoog TR, Jabir MS, Ibraheem S, Jawad SF, Hamzah SS, Sulaiman GM, Mohammed HA, Khan RA. Bacterial extracellular vesicles induced oxidative stress and mitophagy through mTOR pathways in colon cancer cells, HT-29: Implications for bioactivity. Biochim Biophys Acta Mol Cell Res. 2023;1870(6):119486. doi: 10.1016/j.bbamcr.2023.119486. PubMed PMID: 37172765.
93. Gujrati V, Prakash J, Malekzadeh-Najafabadi J, Stiel A, Klemm U, Mettenleiter G, Aichler M, Walch A, Ntziachristos V. Bioengineered bacterial vesicles as biological nano-heaters for optoacoustic imaging. Nat Commun. 2019;10(1):1114. doi: 10.1038/s41467-019-09034-y. PubMed PMID: 30846699; PMCID: PMC6405847.
94. Mi Z, Yao Q, Qi Y, Zheng J, Liu J, Liu Z, Tan H, Ma X, Zhou W, Rong P. Salmonella-mediated blood‒brain barrier penetration, tumor homing and tumor microenvironment regulation for enhanced chemo/bacterial glioma therapy. Acta Pharm Sin B. 2023;13(2):819-33. doi: 10.1016/j.apsb.2022.09.016. PubMed PMID: 36873179; PMCID: PMC9978951.
95. Kashyap D, Panda M, Baral B, Varshney N, R S, Bhandari V, Parmar HS, Prasad A, Jha HC. Outer Membrane Vesicles: An Emerging Vaccine Platform. Vaccines (Basel). 2022;10(10). doi: 10.3390/vaccines10101578. PubMed PMID: 36298443; PMCID: PMC9610665.
96. Jalalifar S, Morovati Khamsi H, Hosseini-Fard SR, Karampoor S, Bajelan B, Irajian G, Mirzaei R. Emerging role of microbiota derived outer membrane vesicles to preventive, therapeutic and diagnostic proposes. Infect Agent Cancer. 2023;18(1):3. doi: 10.1186/s13027-023-00480-4. PubMed PMID: 36658631; PMCID: PMC9850788.
97. Li Y, Wu J, Qiu X, Dong S, He J, Liu J, Xu W, Huang S, Hu X, Xiang DX. Bacterial outer membrane vesicles-based therapeutic platform eradicates triple-negative breast tumor by combinational photodynamic/chemo-/immunotherapy. Bioact Mater. 2023;20:548-60. doi: 10.1016/j.bioactmat.2022.05.037. PubMed PMID: 35846843; PMCID: PMC9253654.
98. Najafi S, Majidpoor J, Mortezaee K. Extracellular vesicle-based drug delivery in cancer immunotherapy. Drug Deliv Transl Res. 2023;13(11):2790-806. doi: 10.1007/s13346-023-01370-3. PubMed PMID: 37261603; PMCID: PMC10234250.
99. Won S, Lee C, Bae S, Lee J, Choi D, Kim MG, Song S, Lee J, Kim E, Shin H, Basukala A, Lee TR, Lee DS, Gho YS. Mass-produced gram-negative bacterial outer membrane vesicles activate cancer antigen-specific stem-like CD8(+) T cells which enables an effective combination immunotherapy with anti-PD-1. J Extracell Vesicles. 2023;12(8):e12357. doi: 10.1002/jev2.12357. PubMed PMID: 37563797; PMCID: PMC10415594.
100. Park KS, Svennerholm K, Crescitelli R, Lasser C, Gribonika I, Lotvall J. Synthetic bacterial vesicles combined with tumour extracellular vesicles as cancer immunotherapy. J Extracell Vesicles. 2021;10(9):e12120. doi: 10.1002/jev2.12120. PubMed PMID: 34262675; PMCID: PMC8254025.
101. Chen L, Ma X, Liu W, Hu Q, Yang H. Targeting Pyroptosis through Lipopolysaccharide-Triggered Noncanonical Pathway for Safe and Efficient Cancer Immunotherapy. Nano Lett. 2023;23(18):8725-33. doi: 10.1021/acs.nanolett.3c02728. PubMed PMID: 37695255.
102. Jiang S, Fu W, Wang S, Zhu G, Wang J, Ma Y. Bacterial Outer Membrane Vesicles Loaded with Perhexiline Suppress Tumor Development by Regulating Tumor-Associated Macrophages Repolarization in a Synergistic Way. Int J Mol Sci. 2023;24(13). doi: 10.3390/ijms241311222. PubMed PMID: 37446401; PMCID: PMC10342243.
103. Kuerban K, Gao X, Zhang H, Liu J, Dong M, Wu L, Ye R, Feng M, Ye L. Doxorubicin-loaded bacterial outer-membrane vesicles exert enhanced anti-tumor efficacy in non-small-cell lung cancer. Acta Pharm Sin B. 2020;10(8):1534-48. doi: 10.1016/j.apsb.2020.02.002. PubMed PMID: 32963948; PMCID: PMC7488491.
104. Guo Q, Li X, Zhou W, Chu Y, Chen Q, Zhang Y, Li C, Chen H, Liu P, Zhao Z, Wang Y, Zhou Z, Luo Y, Li C, You H, Song H, Su B, Zhang T, Sun T, Jiang C. Sequentially Triggered Bacterial Outer Membrane Vesicles for Macrophage Metabolism Modulation and Tumor Metastasis Suppression. ACS Nano. 2021;15(8):13826-38. doi: 10.1021/acsnano.1c05613. PubMed PMID: 34382768.
105. Rezaei Adriani R, Mousavi Gargari SL, Bakherad H, Amani J. Anti-EGFR bioengineered bacterial outer membrane vesicles as targeted immunotherapy candidate in triple-negative breast tumor murine model. Sci Rep. 2023;13(1):16403. doi: 10.1038/s41598-023-43762-y. PubMed PMID: 37775519; PMCID: PMC10541432.
106. Li Y, Zhao R, Cheng K, Zhang K, Wang Y, Zhang Y, Li Y, Liu G, Xu J, Xu J, Anderson GJ, Shi J, Ren L, Zhao X, Nie G. Bacterial Outer Membrane Vesicles Presenting Programmed Death 1 for Improved Cancer Immunotherapy via Immune Activation and Checkpoint Inhibition. ACS Nano. 2020;14(12):16698-711. doi: 10.1021/acsnano.0c03776. PubMed PMID: 33232124.
107. Gujrati V, Kim S, Kim SH, Min JJ, Choy HE, Kim SC, Jon S. Bioengineered bacterial outer membrane vesicles as cell-specific drug-delivery vehicles for cancer therapy. ACS Nano. 2014;8(2):1525-37. doi: 10.1021/nn405724x. PubMed PMID: 24410085.
108. Chen Q, Bai H, Wu W, Huang G, Li Y, Wu M, Tang G, Ping Y. Bioengineering Bacterial Vesicle-Coated Polymeric Nanomedicine for Enhanced Cancer Immunotherapy and Metastasis Prevention. Nano Lett. 2020;20(1):11-21. doi: 10.1021/acs.nanolett.9b02182. PubMed PMID: 31858807.
109. Jiang G, Xiang Z, Fang Q. Engineering magnetotactic bacteria MVs to synergize chemotherapy, ferroptosis and immunotherapy for augmented antitumor therapy. Nanoscale Horiz. 2023;8(8):1062-72. doi: 10.1039/d3nh00061c. PubMed PMID: 37306000.
110. Cui C, He Q, Wang J, Kang J, Ma W, Nian Y, Sun Z, Weng H. Targeted miR-34a delivery with PD1 displayed bacterial outer membrane vesicles-coated zeolitic imidazolate framework nanoparticles for enhanced tumor therapy. Int J Biol Macromol. 2023;247:125692. doi: 10.1016/j.ijbiomac.2023.125692. PubMed PMID: 37414322.
111. Luo ZW, Xia K, Liu YW, Liu JH, Rao SS, Hu XK, Chen CY, Xu R, Wang ZX, Xie H. Extracellular Vesicles from Akkermansia muciniphila Elicit Antitumor Immunity Against Prostate Cancer via Modulation of CD8(+) T Cells and Macrophages. Int J Nanomedicine. 2021;16:2949-63. doi: 10.2147/IJN.S304515. PubMed PMID: 33907401; PMCID: PMC8068512.
112. Gurunathan S, Ajmani A, Kim JH. Extracellular nanovesicles produced by Bacillus licheniformis: A potential anticancer agent for breast and lung cancer. Microb Pathog. 2023;185:106396. doi: 10.1016/j.micpath.2023.106396. PubMed PMID: 37863272.
113. Jiang Y, Xu Y, Zheng C, Ye L, Jiang P, Malik S, Xu G, Zhou Q, Zhang M. Acetyltransferase from Akkermansia muciniphila blunts colorectal tumourigenesis by reprogramming tumour microenvironment. Gut. 2023;72(7):1308-18. doi: 10.1136/gutjnl-2022-327853. PubMed PMID: 36754607.
114. Behzadi E, Mahmoodzadeh Hosseini H, Imani Fooladi AA. The inhibitory impacts of Lactobacillus rhamnosus GG-derived extracellular vesicles on the growth of hepatic cancer cells. Microb Pathog. 2017;110:1-6. doi: 10.1016/j.micpath.2017.06.016. PubMed PMID: 28634130.
115. An J, Kim JB, Yang EY, Kim HO, Lee WH, Yang J, Kwon H, Paik NS, Lim W, Kim YK, Moon BI. Bacterial extracellular vesicles affect endocrine therapy in MCF7 cells. Medicine (Baltimore). 2021;100(18):e25835. doi: 10.1097/MD.0000000000025835. PubMed PMID: 33950995; PMCID: PMC8104188.
116. Jang SC, Kim SR, Yoon YJ, Park KS, Kim JH, Lee J, Kim OY, Choi EJ, Kim DK, Choi DS, Kim YK, Park J, Di Vizio D, Gho YS. In vivo kinetic biodistribution of nano-sized outer membrane vesicles derived from bacteria. Small. 2015;11(4):456-61. doi: 10.1002/smll.201401803. PubMed PMID: 25196673.
117. Parada N, Romero-Trujillo A, Georges N, Alcayaga-Miranda F. Camouflage strategies for therapeutic exosomes evasion from phagocytosis. J Adv Res. 2021;31:61-74. doi: 10.1016/j.jare.2021.01.001. PubMed PMID: 34194832; PMCID: PMC8240105.
118. Piffoux M, Volatron J, Silva AKA, Gazeau F. Thinking Quantitatively of RNA-Based Information Transfer via Extracellular Vesicles: Lessons to Learn for the Design of RNA-Loaded EVs. Pharmaceutics. 2021;13(11). doi: 10.3390/pharmaceutics13111931. PubMed PMID: 34834346; PMCID: PMC8617734.
119. Watson DC, Bayik D, Srivatsan A, Bergamaschi C, Valentin A, Niu G, Bear J, Monninger M, Sun M, Morales-Kastresana A, Jones JC, Felber BK, Chen X, Gursel I, Pavlakis GN. Efficient production and enhanced tumor delivery of engineered extracellular vesicles. Biomaterials. 2016;105:195-205. doi: 10.1016/j.biomaterials.2016.07.003. PubMed PMID: 27522254; PMCID: PMC7156278.
120. Wiklander OP, Nordin JZ, O’Loughlin A, Gustafsson Y, Corso G, Mager I, Vader P, Lee Y, Sork H, Seow Y, Heldring N, Alvarez-Erviti L, Smith CI, Le Blanc K, Macchiarini P, Jungebluth P, Wood MJ, Andaloussi SE. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles. 2015;4:26316. doi: 10.3402/jev.v4.26316. PubMed PMID: 25899407; PMCID: PMC4405624.
121. Driedonks T, Jiang L, Carlson B, Han Z, Liu G, Queen SE, Shirk EN, Gololobova O, Liao Z, Nyberg LH, Lima G, Paniushkina L, Garcia-Contreras M, Schonvisky K, Castell N, Stover M, Guerrero-Martin S, Richardson R, Smith B, Machairaki V, Lai CP, Izzi JM, Hutchinson EK, Pate KAM, Witwer KW. Pharmacokinetics and biodistribution of extracellular vesicles administered intravenously and intranasally to Macaca nemestrina. J Extracell Biol. 2022;1(10). doi: 10.1002/jex2.59. PubMed PMID: 36591537; PMCID: PMC9799283.
122. Xu W, Hao X, Li Y, Tang Y, Qiu X, Zhou M, Liu J, Huang S, Liao D, Hu X, Tang T, Wu J, Xiang D. Safe Induction of Acute Inflammation with Enhanced Antitumor Immunity by Hydrogel-Mediated Outer Membrane Vesicle Delivery. Small Methods. 2024:e2301620. doi: 10.1002/smtd.202301620. PubMed PMID: 38343178.
123. Chen J, Tan Q, Yang Z, Jin Y. Engineered extracellular vesicles: potentials in cancer combination therapy. J Nanobiotechnology. 2022;20(1):132. doi: 10.1186/s12951-022-01330-y. PubMed PMID: 35292030; PMCID: PMC8922858.
124. Esmaeili A, Alini M, Baghaban Eslaminejad M, Hosseini S. Engineering strategies for customizing extracellular vesicle uptake in a therapeutic context. Stem Cell Res Ther. 2022;13(1):129. doi: 10.1186/s13287-022-02806-2. PubMed PMID: 35346367; PMCID: PMC8960087.
125. Johnson V, Vasu S, Kumar US, Kumar M. Surface-Engineered Extracellular Vesicles in Cancer Immunotherapy. Cancers (Basel). 2023;15(10). doi: 10.3390/cancers15102838. PubMed PMID: 37345176; PMCID: PMC10216164.
126. Zheng K, Feng Y, Li L, Kong F, Gao J, Kong X. Engineered bacterial outer membrane vesicles: a versatile bacteria-based weapon against gastrointestinal tumors. Theranostics. 2024;14(2):761-87. doi: 10.7150/thno.85917. PubMed PMID: 38169585; PMCID: PMC10758051.
127. Cheng L, Zhang X, Tang J, Lv Q, Liu J. Gene-engineered exosomes-thermosensitive liposomes hybrid nanovesicles by the blockade of CD47 signal for combined photothermal therapy and cancer immunotherapy. Biomaterials. 2021;275:120964. doi: 10.1016/j.biomaterials.2021.120964. PubMed PMID: 34147721.
128. Danilushkina AA, Emene CC, Barlev NA, Gomzikova MO. Strategies for Engineering of Extracellular Vesicles. Int J Mol Sci. 2023;24(17). doi: 10.3390/ijms241713247. PubMed PMID: 37686050; PMCID: PMC10488046.
129. Herrmann IK, Wood MJA, Fuhrmann G. Extracellular vesicles as a next-generation drug delivery platform. Nat Nanotechnol. 2021;16(7):748-59. doi: 10.1038/s41565-021-00931-2. PubMed PMID: 34211166.
130. Pan J, Li X, Shao B, Xu F, Huang X, Guo X, Zhou S. Self-Blockade of PD-L1 with Bacteria-Derived Outer-Membrane Vesicle for Enhanced Cancer Immunotherapy. Adv Mater. 2022;34(7):e2106307. doi: 10.1002/adma.202106307. PubMed PMID: 34859919.
131. Mancini F, Rossi O, Necchi F, Micoli F. OMV Vaccines and the Role of TLR Agonists in Immune Response. Int J Mol Sci. 2020;21(12). doi: 10.3390/ijms21124416. PubMed PMID: 32575921; PMCID: PMC7352230.
132. Gasparini R, Amicizia D, Domnich A, Lai PL, Panatto D. Neisseria meningitidis B vaccines: recent advances and possible immunization policies. Expert Rev Vaccines. 2014;13(3):345-64. doi: 10.1586/14760584.2014.880341. PubMed PMID: 24476428.
133. Masforrol Y, Gil J, Garcia D, Noda J, Ramos Y, Betancourt L, Guirola O, Gonzalez S, Acevedo B, Besada V, Reyes O, Gonzalez LJ. A deeper mining on the protein composition of VA-MENGOC-BC(R): An OMV-based vaccine against N. meningitidis serogroup B and C. Hum Vaccin Immunother. 2017;13(11):2548-60. doi: 10.1080/21645515.2017.1356961. PubMed PMID: 29083947; PMCID: PMC5798414.
134. Liu Y, Defourny KAY, Smid EJ, Abee T. Gram-Positive Bacterial Extracellular Vesicles and Their Impact on Health and Disease. Front Microbiol. 2018;9:1502. doi: 10.3389/fmicb.2018.01502. PubMed PMID: 30038605; PMCID: PMC6046439.
135. Gao X, Feng Q, Wang J, Zhao X. Bacterial outer membrane vesicle-based cancer nanovaccines. Cancer Biol Med. 2022;19(9):1290-300. doi: 10.20892/j.issn.2095-3941.2022.0452. PubMed PMID: 36172794; PMCID: PMC9500226.
136. Wang S, Guo J, Bai Y, Sun C, Wu Y, Liu Z, Liu X, Wang Y, Wang Z, Zhang Y, Hao H. Bacterial outer membrane vesicles as a candidate tumor vaccine platform. Front Immunol. 2022;13:987419. doi: 10.3389/fimmu.2022.987419. PubMed PMID: 36159867; PMCID: PMC9505906.
137. Grandi A, Fantappie L, Irene C, Valensin S, Tomasi M, Stupia S, Corbellari R, Caproni E, Zanella I, Isaac SJ, Ganfini L, Frattini L, Konig E, Gagliardi A, Tavarini S, Sammicheli C, Parri M, Grandi G. Vaccination With a FAT1-Derived B Cell Epitope Combined With Tumor-Specific B and T Cell Epitopes Elicits Additive Protection in Cancer Mouse Models. Front Oncol. 2018;8:481. doi: 10.3389/fonc.2018.00481. PubMed PMID: 30416985; PMCID: PMC6212586.
138. Grandi A, Tomasi M, Zanella I, Ganfini L, Caproni E, Fantappie L, Irene C, Frattini L, Isaac SJ, Konig E, Zerbini F, Tavarini S, Sammicheli C, Giusti F, Ferlenghi I, Parri M, Grandi G. Synergistic Protective Activity of Tumor-Specific Epitopes Engineered in Bacterial Outer Membrane Vesicles. Front Oncol. 2017;7:253. doi: 10.3389/fonc.2017.00253. PubMed PMID: 29164053; PMCID: PMC5681935.
139. Huang W, Shu C, Hua L, Zhao Y, Xie H, Qi J, Gao F, Gao R, Chen Y, Zhang Q, Li W, Yuan M, Ye C, Ma Y. Modified bacterial outer membrane vesicles induce autoantibodies for tumor therapy. Acta Biomater. 2020;108:300-12. doi: 10.1016/j.actbio.2020.03.030. PubMed PMID: 32251780.
140. Cheng K, Zhao R, Li Y, Qi Y, Wang Y, Zhang Y, Qin H, Qin Y, Chen L, Li C, Liang J, Li Y, Xu J, Han X, Anderson GJ, Shi J, Ren L, Zhao X, Nie G. Bioengineered bacteria-derived outer membrane vesicles as a versatile antigen display platform for tumor vaccination via Plug-and-Display technology. Nat Commun. 2021;12(1):2041. doi: 10.1038/s41467-021-22308-8. PubMed PMID: 33824314; PMCID: PMC8024398.
141. Li Y, Ma X, Yue Y, Zhang K, Cheng K, Feng Q, Ma N, Liang J, Zhang T, Zhang L, Chen Z, Wang X, Ren L, Zhao X, Nie G. Rapid Surface Display of mRNA Antigens by Bacteria-Derived Outer Membrane Vesicles for a Personalized Tumor Vaccine. Adv Mater. 2022;34(20):e2109984. doi: 10.1002/adma.202109984. PubMed PMID: 35315546.
142. Liang J, Zhu F, Cheng K, Ma N, Ma X, Feng Q, Xu C, Gao X, Wang X, Shi J, Zhao X, Nie G. Outer Membrane Vesicle-Based Nanohybrids Target Tumor-Associated Macrophages to Enhance Trained Immunity-Related Vaccine-Generated Antitumor Activity. Adv Mater. 2023;35(46):e2306158. doi: 10.1002/adma.202306158. PubMed PMID: 37643537.
143. Diaz-Garrido N, Badia J, Baldoma L. Microbiota-derived extracellular vesicles in interkingdom communication in the gut. J Extracell Vesicles. 2021;10(13):e12161. doi: 10.1002/jev2.12161. PubMed PMID: 34738337; PMCID: PMC8568775.
144. Suri K, D’Souza A, Huang D, Bhavsar A, Amiji M. Bacterial extracellular vesicle applications in cancer immunotherapy. Bioact Mater. 2023;22:551-66. doi: 10.1016/j.bioactmat.2022.10.024. PubMed PMID: 36382022; PMCID: PMC9637733.
145. Paolini L, Monguió‐Tortajada M, Costa M, Antenucci F, Barilani M, Clos‐Sansalvador M, Andrade AC, Driedonks TAP, Giancaterino S, Kronstadt SM, Mizenko RR, Nawaz M, Osteikoetxea X, Pereira C, Shrivastava S, Boysen AT, van de Wakker SI, van Herwijnen MJC, Wang X, Watson DC, Gimona M, Kaparakis‐Liaskos M, Konstantinov K, Lim SK, Meisner‐Kober N, Stork M, Nejsum P, Radeghieri A, Rohde E, Touzet N, Wauben MHM, Witwer KW, Bongiovanni A, Bergese P. Large‐scale production of extracellular vesicles: Report on the “massivEVs” ISEV workshop. J Extracell Biol. 2022;1(10):e63. doi: 10.1002/jex2.63.
146. Wen M, Wang J, Ou Z, Nie G, Chen Y, Li M, Wu Z, Xiong S, Zhou H, Yang Z, Long G, Su J, Liu H, Jing Y, Wen Z, Fu Y, Zhou T, Xie H, Guan W, Sun X, Wang Z, Wang J, Chen X, Jiang L, Qin X, Xue Y, Huang M, Huang X, Pan R, Zhen H, Du Y, Li Q, Huang X, Wu Y, Wang P, Zhao K, Situ B, Hu X, Zheng L. Bacterial extracellular vesicles: A position paper by the microbial vesicles task force of the Chinese society for extracellular vesicles. Interdisciplinary Medicine. 2023;1(3):e20230017. doi: 10.1002/inmd.20230017.
147. Lener T, Gimona M, Aigner L, Borger V, Buzas E, Camussi G, Chaput N, Chatterjee D, Court FA, Del Portillo HA, O’Driscoll L, Fais S, Falcon-Perez JM, Felderhoff-Mueser U, Fraile L, Gho YS, Gorgens A, Gupta RC, Hendrix A, Hermann DM, Hill AF, Hochberg F, Horn PA, de Kleijn D, Kordelas L, Kramer BW, Kramer-Albers EM, Laner-Plamberger S, Laitinen S, Leonardi T, Lorenowicz MJ, Lim SK, Lotvall J, Maguire CA, Marcilla A, Nazarenko I, Ochiya T, Patel T, Pedersen S, Pocsfalvi G, Pluchino S, Quesenberry P, Reischl IG, Rivera FJ, Sanzenbacher R, Schallmoser K, Slaper-Cortenbach I, Strunk D, Tonn T, Vader P, van Balkom BW, Wauben M, Andaloussi SE, Thery C, Rohde E, Giebel B. Applying extracellular vesicles based therapeutics in clinical trials - an ISEV position paper. J Extracell Vesicles. 2015;4:30087. doi: 10.3402/jev.v4.30087. PubMed PMID: 26725829; PMCID: PMC4698466.
148. Clemmens H, Lambert DW. Extracellular vesicles: translational challenges and opportunities. Biochem Soc Trans. 2018;46(5):1073-82. doi: 10.1042/BST20180112. PubMed PMID: 30242120.
149. Pirolli NH, Reus LSC, Mamczarz Z, Khan S, Bentley WE, Jay SM. High performance anion exchange chromatography purification of probiotic bacterial extracellular vesicles enhances purity and anti-inflammatory efficacy. Biotechnol Bioeng. 2023;120(11):3368-80. doi: 10.1002/bit.28522. PubMed PMID: 37555379; PMCID: PMC10592193.
150. Piroli NH, Reus LSC, Mamczarz Z, Khan S, Bentley WE, Jay SM. High performance anion exchange chromatography purification of probiotic bacterial extracellular vesicles enhances purity and anti-inflammatory efficacy. bioRxiv. 2023. doi: 10.1101/2023.05.01.538917. PubMed PMID: 37205369; PMCID: PMC10187247.
151. Dong L, Zieren RC, Horie K, Kim CJ, Mallick E, Jing Y, Feng M, Kuczler MD, Green J, Amend SR, Witwer KW, de Reijke TM, Cho YK, Pienta KJ, Xue W. Comprehensive evaluation of methods for small extracellular vesicles separation from human plasma, urine and cell culture medium. J Extracell Vesicles. 2020;10(2):e12044. doi: 10.1002/jev2.12044. PubMed PMID: 33489012; PMCID: PMC7810129.
152. Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, Dingli F, Loew D, Tkach M, Thery C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A. 2016;113(8):E968-77. doi: 10.1073/pnas.1521230113. PubMed PMID: 26858453; PMCID: PMC4776515.
153. Toth EA, Turiak L, Visnovitz T, Cserep C, Mazlo A, Sodar BW, Forsonits AI, Petovari G, Sebestyen A, Komlosi Z, Drahos L, Kittel A, Nagy G, Bacsi A, Denes A, Gho YS, Szabo-Taylor KE, Buzas EI. Formation of a protein corona on the surface of extracellular vesicles in blood plasma. J Extracell Vesicles. 2021;10(11):e12140. doi: 10.1002/jev2.12140. PubMed PMID: 34520123; PMCID: PMC8439280.
154. Consortium E-T, Van Deun J, Mestdagh P, Agostinis P, Akay O, Anand S, Anckaert J, Martinez ZA, Baetens T, Beghein E, Bertier L, Berx G, Boere J, Boukouris S, Bremer M, Buschmann D, Byrd JB, Casert C, Cheng L, Cmoch A, Daveloose D, De Smedt E, Demirsoy S, Depoorter V, Dhondt B, Driedonks TA, Dudek A, Elsharawy A, Floris I, Foers AD, Gartner K, Garg AD, Geeurickx E, Gettemans J, Ghazavi F, Giebel B, Kormelink TG, Hancock G, Helsmoortel H, Hill AF, Hyenne V, Kalra H, Kim D, Kowal J, Kraemer S, Leidinger P, Leonelli C, Liang Y, Lippens L, Liu S, Lo Cicero A, Martin S, Mathivanan S, Mathiyalagan P, Matusek T, Milani G, Monguio-Tortajada M, Mus LM, Muth DC, Nemeth A, Nolte-’t Hoen EN, O’Driscoll L, Palmulli R, Pfaffl MW, Primdal-Bengtson B, Romano E, Rousseau Q, Sahoo S, Sampaio N, Samuel M, Scicluna B, Soen B, Steels A, Swinnen JV, Takatalo M, Thaminy S, Thery C, Tulkens J, Van Audenhove I, van der Grein S, Van Goethem A, van Herwijnen MJ, Van Niel G, Van Roy N, Van Vliet AR, Vandamme N, Vanhauwaert S, Vergauwen G, Verweij F, Wallaert A, Wauben M, Witwer KW, Zonneveld MI, De Wever O, Vandesompele J, Hendrix A. EV-TRACK: transparent reporting and centralizing knowledge in extracellular vesicle research. Nat Methods. 2017;14(3):228-32. doi: 10.1038/nmeth.4185. PubMed PMID: 28245209.
155. Arab T, Mallick ER, Huang Y, Dong L, Liao Z, Zhao Z, Gololobova O, Smith B, Haughey NJ, Pienta KJ, Slusher BS, Tarwater PM, Tosar JP, Zivkovic AM, Vreeland WN, Paulaitis ME, Witwer KW. Characterization of extracellular vesicles and synthetic nanoparticles with four orthogonal single-particle analysis platforms. J Extracell Vesicles. 2021;10(6):e12079. doi: 10.1002/jev2.12079. PubMed PMID: 33850608; PMCID: PMC8023330.
156. Webber J, Clayton A. How pure are your vesicles? J Extracell Vesicles. 2013;2. doi: 10.3402/jev.v2i0.19861. PubMed PMID: 24009896; PMCID: PMC3760653.
157. Balhuizen MD, Veldhuizen EJA, Haagsman HP. Outer Membrane Vesicle Induction and Isolation for Vaccine Development. Front Microbiol. 2021;12:629090. doi: 10.3389/fmicb.2021.629090. PubMed PMID: 33613498; PMCID: PMC7889600.
158. Patel DB, Luthers CR, Lerman MJ, Fisher JP, Jay SM. Enhanced extracellular vesicle production and ethanol-mediated vascularization bioactivity via a 3D-printed scaffold-perfusion bioreactor system. Acta Biomater. 2019;95:236-44. doi: 10.1016/j.actbio.2018.11.024. PubMed PMID: 30471476; PMCID: PMC6531369.
159. Watson DC, Johnson S, Santos A, Yin M, Bayik D, Lathia JD, Dwidar M. Scalable Isolation and Purification of Extracellular Vesicles from Escherichia coli and Other Bacteria. J Vis Exp. 2021(176). doi: 10.3791/63155. PubMed PMID: 34723953; PMCID: PMC8729794.
160. Hong J, Dauros-Singorenko P, Whitcombe A, Payne L, Blenkiron C, Phillips A, Swift S. Analysis of the Escherichia coli extracellular vesicle proteome identifies markers of purity and culture conditions. J Extracell Vesicles. 2019;8(1):1632099. doi: 10.1080/20013078.2019.1632099. PubMed PMID: 31275533; PMCID: PMC6598517.
161. Yu YJ, Wang XH, Fan GC. Versatile effects of bacterium-released membrane vesicles on mammalian cells and infectious/inflammatory diseases. Acta Pharmacol Sin. 2018;39(4):514-33. doi: 10.1038/aps.2017.82. PubMed PMID: 28858295; PMCID: PMC5888691.
162. Chen X, Li P, Luo B, Song C, Wu M, Yao Y, Wang D, Li X, Hu B, He S, Zhao Y, Wang C, Yang X, Hu J. Surface Mineralization of Engineered Bacterial Outer Membrane Vesicles to Enhance Tumor Photothermal/Immunotherapy. ACS Nano. 2024;18(2):1357-70. doi: 10.1021/acsnano.3c05714. PubMed PMID: 38164903.
163. Liu L, Liang L, Yang C, Zhou Y, Chen Y. Extracellular vesicles of Fusobacterium nucleatum compromise intestinal barrier through targeting RIPK1-mediated cell death pathway. Gut Microbes. 2021;13(1):1-20. doi: 10.1080/19490976.2021.1902718. PubMed PMID: 33769187; PMCID: PMC8007154.
164. Taylor ML, Giacalone AG, Amrhein KD, Wilson RE, Jr., Wang Y, Huang X. Nanomaterials for Molecular Detection and Analysis of Extracellular Vesicles. Nanomaterials (Basel). 2023;13(3). doi: 10.3390/nano13030524. PubMed PMID: 36770486; PMCID: PMC9920192.
165. Zhuo Z, Wang J, Luo Y, Zeng R, Zhang C, Zhou W, Guo K, Wu H, Sha W, Chen H. Targeted extracellular vesicle delivery systems employing superparamagnetic iron oxide nanoparticles. Acta Biomater. 2021;134:13-31. doi: 10.1016/j.actbio.2021.07.027. PubMed PMID: 34284151.
166. Liu X, Xiao C, Xiao K. Engineered extracellular vesicles-like biomimetic nanoparticles as an emerging platform for targeted cancer therapy. J Nanobiotechnology. 2023;21(1):287. doi: 10.1186/s12951-023-02064-1. PubMed PMID: 37608298; PMCID: PMC10463632.
167. Monnier M, Paolini L, Vinatier E, Mantovani A, Delneste Y, Jeannin P. Antitumor strategies targeting macrophages: the importance of considering the differences in differentiation/polarization processes between human and mouse macrophages. J Immunother Cancer. 2022;10(10). doi: 10.1136/jitc-2022-005560. PubMed PMID: 36270732; PMCID: PMC9594518.
168. Steeghs L, Keestra AM, van Mourik A, Uronen-Hansson H, van der Ley P, Callard R, Klein N, van Putten JP. Differential activation of human and mouse Toll-like receptor 4 by the adjuvant candidate LpxL1 of Neisseria meningitidis. Infect Immun. 2008;76(8):3801-7. doi: 10.1128/IAI.00005-08. PubMed PMID: 18490457; PMCID: PMC2493235.
169. Gao J, Su Y, Wang Z. Engineering bacterial membrane nanovesicles for improved therapies in infectious diseases and cancer. Adv Drug Deliv Rev. 2022;186:114340. doi: 10.1016/j.addr.2022.114340. PubMed PMID: 35569561; PMCID: PMC9899072.
170. Lee TY, Kim CU, Bae EH, Seo SH, Jeong DG, Yoon SW, Chang KT, Kim YS, Kim SH, Kim DJ. Outer membrane vesicles harboring modified lipid A moiety augment the efficacy of an influenza vaccine exhibiting reduced endotoxicity in a mouse model. Vaccine. 2017;35(4):586-95. doi: 10.1016/j.vaccine.2016.12.025. PubMed PMID: 28024958; PMCID: PMC7115551.
171. Gon Y, Maruoka S, Inoue T, Kuroda K, Yamagishi K, Kozu Y, Shikano S, Soda K, Lotvall J, Hashimoto S. Selective release of miRNAs via extracellular vesicles is associated with house-dust mite allergen-induced airway inflammation. Clin Exp Allergy. 2017;47(12):1586-98. doi: 10.1111/cea.13016. PubMed PMID: 28859242.
172. Kim YS, Choi EJ, Lee WH, Choi SJ, Roh TY, Park J, Jee YK, Zhu Z, Koh YY, Gho YS, Kim YK. Extracellular vesicles, especially derived from Gram-negative bacteria, in indoor dust induce neutrophilic pulmonary inflammation associated with both Th1 and Th17 cell responses. Clin Exp Allergy. 2013;43(4):443-54. doi: 10.1111/cea.12085. PubMed PMID: 23517040.
173. Qing S, Lyu C, Zhu L, Pan C, Wang S, Li F, Wang J, Yue H, Gao X, Jia R, Wei W, Ma G. Biomineralized Bacterial Outer Membrane Vesicles Potentiate Safe and Efficient Tumor Microenvironment Reprogramming for Anticancer Therapy. Adv Mater. 2020;32(47):e2002085. doi: 10.1002/adma.202002085. PubMed PMID: 33015871.
Submitted December 18, 2023 | Accepted March 28, 2024 | Published April 23, 2024
Copyright © 2024 The Authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License.