Nanak S. Dhillon1,2, Nayeon Jeon1,2, Umut A. Gurkan3, Anirban Sen Gupta4, Robert A. Bonomo2,5, Lawrence F. Drummy6, Mei Zhang4, Mark R. Chance1,2,4
1Department of Nutrition, School of Medicine, Case Western Reserve University, Cleveland, OH
2Center for Proteomics and Bioinformatics, School of Medicine, Case Western Reserve University, Cleveland, Ohio
3Department of Mechanical and Aerospace Engineering, Case School of Engineering, Case Western Reserve University, Cleveland, Ohio
4Department of Biomedical Engineering, School of Medicine, Case School of Engineering, Case Western Reserve University, Cleveland, Ohio
5Louis Stokes Cleveland Department of Veterans Affairs Medical Center; Case Western Reserve University, Cleveland, OH; VAMC Center for Antimicrobial Resistance and Epidemiology (Case VA CARES); Departments of Medicine, Pharmacology, Molecular Biology and Microbiology, and Biochemistry, Case Western Reserve University, Cleveland, Ohio
6Materials and Manufacturing Directorate, Air Force Research Laboratory, Dayton, Ohio
Mark Chance, PhD
mark.chance@case.edu
Dhillon NS, Jeon N, Gurka UA, Gupta AS, Bonomo RA, Drummy LF, Zhang M, Chance MR. Military Medicine and Medical Research as a Source of Inspiration and Innovation to Solve National Security and Health Challenges in the 21st Century. Pathogens and Immunity. 2023;8(1):51–63.
10.20411/pai.v8i1.596
The history of military medicine and research is rife with examples of novel treatments and new approaches to heal and cure soldiers and others impacted by war’s devastation. In the 21st century, new threats, like climate change, are combined with traditional threats, like geopolitical conflict, to create novel challenges for our strategic interests. Extreme and inaccessible environments provide heightened risks for warfighter exposure to dangerous bacteria, viruses, and fungi, as well as exposure to toxic substances and extremes of temperature, pressure, or both providing threats to performance and eroding resilience. Back home, caring for our veterans is also a healthcare priority, and the diseases of veterans increasingly overlap with the health needs of an aging society. These trends of climate change, politics, and demographics suggest performance evaluation and resilience planning and response are critical to assuring both warfighter performance and societal health. The Cleveland ecosystem, comprising several hospitals, a leading University, and one of the nation’s larger Veteran’s Health Administration systems, is ideal for incubating and understanding the response to these challenges. In this review, we explore the interconnections of collaborations between Defense agencies, particularly Air Force and Army and academic medical center-based investigators to drive responses to the national health security challenges facing the United States and the world.
Health research; fatigue and stress; sensors; human performance and resilience
Department of Defense (DOD)-related biomedical research is a large and growing enterprise, and with the recent formation of the Advanced Research Projects Agency for Health, it is expanding its potential for additional funding of relevant projects [1, 2]. Academic medical centers are receiving tens of billions of dollars in federal funding to find cures and treatments for diseases, and programs are being developed to help investigators learn to transfer these innovations from the laboratory to the hands of patients and providers [3, 4]. In particular, the US National Biodefense Strategy (Oct 2022) recognizes that pathogens are global risks, and that enhancing resilience means strengthening global health defense to protect the nation in the same ways we develop and project conventional defenses. We cannot start to fight at the border because that will be too late. A DOD-inspired symposium was recently held at Case Western Reserve University in Cleveland, Ohio, where field-leading experts from all over the country were able to convene and discuss some of the challenges and innovations outlined in this paper.
In examining the potential future needs for protecting the United States and its warfighters and their support systems, a few obvious trends are settling in place. First, the trend of climate change will increase the frequency of serious infections, extend the ranges of some pathogen-induced diseases, and escalate the severity of heat-related threats to warfighters and society. Second, current geopolitical conflict trends suggest that many battles are fought in unfamiliar, extreme, or challenging environments where monitoring performance in real time and enhancing resilience may be critical to success. Lastly, an aging US population and an aging veteran population increasingly merge the needs of veterans and society at large. Integrating disparate federally supported research efforts can only help to improve efficiencies and increase opportunities for collaborations to permit faster and further progress toward health solutions. In this review, the authors outline their individual and joint efforts to respond to these emerging challenges. In total, these activities intend to measure and optimize human performance, and develop warfighter and societal resilience, both in advance of threats and in response to them.
To understand the future of military medicine and the value of biomedical research to the welfare of soldiers and veterans, we must first understand its past. Critical to understand is the concept of War Pestilence, in which infectious diseases claimed the lives of many troops. In fact, diseases such as malaria, typhoid, plague, dysentery, and cholera caused approximately 7 times as many deaths as combat-related injuries in the period prior to the 19th century [5, 6]. In response to this obvious need, there is an extensive history of wound and infection care dating back to over 4,000 years ago. In this period, according to stone carvings, Sumerians used beer and milk to wash wounds before wrapping them [7]. Approximately 3,500 years ago, the Egyptians used the antibacterial properties of honey to treat wounds. Approximately 2,800 years ago, the Greeks used hot water and wine to clean wounds [8]. As gunpowder became commonly used in 14th and 15th century Europe, wounds became much more complex to treat. In the American Civil War, the recent development of general anesthesia played a crucial role in delaying amputation to reduce the effect of wound shock, and bromine was used to prevent hospital gangrene [9]. An estimated 60,000 amputations were performed in the newly developed pavilion-type hospitals [10].
In World War I, the critical developments of debridement, ABO-compatible blood transfusions, and topical antiseptics saved many lives [11]. Additionally, an important observation on the types of bacteria found in wounds in World War I, is that these pathogens were primarily Gram-positive, and relatively simple to understand. In World War II, sulfanilamide and penicillin were used to treat the majority of bacterial infections. In the Korean War, the innovation of Mobile Army Surgical Hospitals (MASH) and the use of helicopters for rapid evacuation of casualties helped save many lives [12]. Towards the end of the Korean war, penicillins and streptomycin were used for bacterial infections, and were particularly effective within the “golden hour,” or first 60 minutes, but some resistance began to be detected.
By the Vietnam War, there was a rapid evolution seen in the complexity of bacterial pathogens, as a mixture of both Gram-positive and Gram-negative bacteria became prevalent [13]. This rapid evolution foreshadowed what would then happen in Iraq and Afghanistan, with even more complex bacteria becoming predominant (ie, Acinetobacter spp.) Entering the modern day, there are many multi-drug resistant (MDR) bacterial strains that are emerging [14]. The Military Infectious Diseases Research Program (MIDRP) accordingly has a goal of helping the DOD prevent, predict, and treat these evolving disease threats. Vector-borne and zoonotic threats have become increasingly threatening to troops [15]. In modern warfare, explosive injuries and resulting infections are of key concern.
All the above provide concrete examples of military-driven innovation that became standard practice for use in society shortly thereafter. Going forward, the focus of infectious disease programs (eg, MIDRP) includes wound infections, endemic diarrheal diseases, viral diseases including HIV, dengue virus, and emerging infectious diseases [16]. By predicting, preventing, and treating these threats, more warfighters can stay on the battlefield, and more support personnel can continue maintaining combat power and increasing battle success rates.
Table 1. Three Thousand Year Timeline of War Periods and Corresponding Medical Innovations
|
Common source of infectious diseases |
Spear wounds |
Gunshot wounds |
Wound shock |
Gram positive bacteria |
Gram positive bacteria |
Bacterial resistance detected |
Mix of gram positive, negative bacteria |
Multidrug resistant (MDR) bacteria |
|
War/Time Period |
Civilizations in Centuries BC |
Europe 14th |
Civil War |
WWI |
WWII |
Korean War |
Vietnam War |
Modern Day |
|
Innovation |
Alcohol, hot water, honey |
Amputation |
General anesthesia, bromine. Pavilion hospitals. |
Debridement, ABO blood transfusion, topical antiseptics, x-rays |
Sulfanilamide, penicillin, masks and sterile instruments |
Mobile Army Surgical Hospitals (MASH), streptomycin |
Rapid helicopter evacuation and better surgeon training |
Military Infectious Disease Research Program (MIDRP) |
A hypothesis developed from the work of Arturo Casadevall may explain why we are seeing a rapid evolution in disease threats over a relatively short period of time [17]. The hypothesis is that climate change is playing a pivotal role in accelerating the evolution of infectious diseases by increasing the frequency of cross-species transmission [18]. As many environments are becoming less suitable for animal and human life due to natural disasters, deforestation, and temperature shifts, the environments of animals and humans are being pushed closer together. As animal-animal and human-animal interactions become more frequent, vector-borne diseases are having more opportunities to travel between species. This increased transmission is allowing for re-emergence of previously diminished infection threats, the evolution of infection threats, and the emergence of entirely new threats like COVID-19 [19]. Thus, the need to increase prioritization of vaccines and other means of subverting viral, bacterial, fungal, and parasitic pathogens is apparent. Collaboration of global health and epidemiology departments coupled with immunology and pharmacology research programs are prime candidates for inclusion on relevant research teams. Host defense and immunity will play increasing roles in disease management and treatment. The development of warfighter agents will also overlap with civilian needs, emphasizing the logic of translational initiatives that benefit both the DOD and society at large.
Performance monitoring of warfighters raises the question: What measurements are actionable for real-world warfighter situations? The question depends on the application of course, but models for examining performance can be derived from high stress and fatigue situations such as combat aviation. Damato and colleagues have suggested that “cognitive fatigue” is an outcome of tactical aviation-induced systemic inflammation [20]. They hypothesized the relationships between serum analytes and “brain fog” in aviation, which could be potential biomarkers of fatigue and sleepiness for tactical aviators. For this study, brain fog was defined as both fatigue and sleepiness, which was repeatedly reported by instructor pilots who fly multiple flights per day with student pilots. This is a constant threat for aviators, particularly because it is the “likely cause of the next mishap,” endangering aviator safety and reducing human performance. Researchers must identify the quantitative physiological biomarkers that correspond to increased levels of fatigue to prevent accidents and enhance flight performance. For tactical aviators, both fatigue and sleepiness appear upon increased levels of proinflammatory cytokines in systemic and central nervous systems. Due to frequent exposure to high-performance aviation events, recurring synthesis and release of proinflammatory cytokines may induce cognitive fatigue in Air Force pilots.
To confirm potential relationships between the biochemical mechanisms and the rise of fatigue for pilots, T-6A Texan II instructor pilots who were scheduled to have more than 2 flights during data collection week were examined. Damato et al made a physical assessment and general, physical, and mental fatigue, motivation and activity, as well as urine, serum, and blood chemistry analysis. Eleven serum analytes were significantly correlated with increased levels of general fatigue, including MCP-1 and MCP-4. These monocyte chemoattractant proteins play a key role in recruiting inflammatory cells to inflammation sites, by activating cell migration signaling pathways [21]. Beyond general fatigue, they have also been implicated in neuroinflammatory and cardiovascular diseases. The data from Damato et al provides both potential biomarkers, as well as mechanistic information on fatigue and stress. However, there is a gap in correlating these novel molecular markers with traditional performance measures that can only be filled by relevant clinical studies. Over time, these biomarker and clinical data will be merged with traditional measures of fitness and readiness and further coupled to additional physiologic data (like blood pressure, heart rate, etc.) to provide multi-modal performance “instruments” to help assist in flight readiness decision making. Further, the need for performance sensing is also significant in the civilian community, as sensors for fatigue could have significant utility for long-haul truck drivers, medical professionals, athletes, airline pilots, and others who experience high occupational stress, fatigue, or both. Real-time performance data can be applied to prevent fatigue-related traffic accidents, improve work efficiency, reduce occurrence of workplace injury, monitor injury recovery, and potentially much more. Performance monitoring is clearly a health challenge for not only the military, but also society at large, and with continued development of these devices that sense performance biomarkers such as lactate, there are opportunities to save lives and improve societal health [22].
A primary focus of laboratory research for military medicine is to develop sensors for monitoring the health, safety, and performance of military personnel. The current status of diagnosis and monitoring using micro-technologies with point-of-care performance has recently been reviewed for COVID-19, but it has been noted that these devices may have many more profound disease applications as well [23]. An and colleagues have used anemia detection and hemoglobin variant identification as examples, as these are both key indicators of malaria and hemoglobinopathies such as sickle cell disease [24]. Particularly in low-resource settings where these diseases are also quite prevalent, the main challenges are cost, laboratory infrastructure, trained personnel, result interpretation, and data management [25]. Even in settings with ample resources, many of these challenges persist to a considerable extent. Nevertheless, a trend is emerging where physiologic types of signals (heart rate, blood pressure etc.) generated from wearables like rings, bands, or watches can now be supplemented with direct molecular measures of substances of interest in body fluids, for example, lactate, cortisol, or neuropeptide-Y, which reflect multiple potential health and disease states [26]. Thus, the limited prediction variables currently provided by physiologic monitoring will be revolutionized by the addition of specific molecular markers for understanding pathologies of interest.
Radwan et al have published on developments in electrochemical molecular biosensors and highlighted the importance of these sensors being highly specific to differentiate compounds with similar structures, as well as highly sensitive to accurately quantify the low levels found in biological analytes [27]. Additionally, these sensors must be simple to use and able to be miniaturized, give readings in real time, and communicate with other devices in a smart system or body-area network. Microneedles properly layered and prepared are for detecting substances such as cortisol, whose levels in serum parallel those seen in interstitial fluid [28]. Nanostructures deposited onto the surface of microneedles allow for the attachment of specific recognition elements that are capable of accurately sensing stress marker levels. These technologies have obvious applications for both warfighters and society, ranging from diagnostics in low-resource settings to real-time sensing of individuals, from athletes to patients in clinical trials.

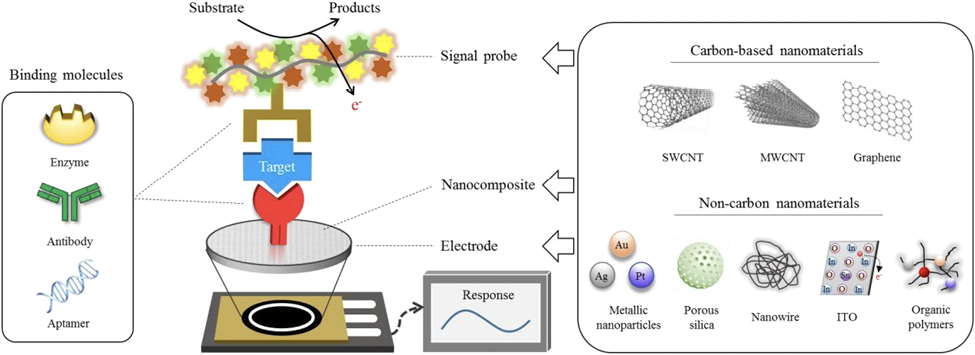
Figure 1. Components of an electrochemical biosensor. Electrochemical biosensors must be specific to their target to accurately characterize amidst signal noise as well as highly sensitive to precisely quantify the µM level concentrations found in biological samples. The necessary specificity and sensitivity are achieved by careful selection of surface molecular receptors and adjustment of enzymatic activity. Carbon-based electrodes offer high surface area to volume ratio, as well as rapid electron transfer capability, making them suitable for achieving miniaturization and real-time data output. [29]. Image Source: Cho IH, Kim DH, Park S. In Biomaterials Research. Springer Nature. Feb 4, 2020. CC BY 4.0.
Warfighter injuries like trauma and bleeding are immediate risks of combat, and the availability of blood and blood products is critical to saving injured personnel. The management of uncontrolled hemorrhage is crucial at warfare scenes. Acidosis, trauma-induced coagulopathy (TIC), and hypothermia, called the “Triangle of Death,” feed into each other to induce the death of soldiers [30]. These are worthy of attention, especially because they are the major causes of preventable mortality. As robust clinical research indicates the importance of early blood transfusion to reduce mortality, battlefield blood transfusion plays a vital role in the prevention trauma-induced hemorrhage and coagulopathy [31]. For transfusion on the battlefield, Anirban Sen Gupta and colleagues are currently adapting the Massive Transfusion Protocol that utilizes a 1:1:1 ratio mixture of platelet, red blood cells, and plasma. However, blood transfusion at military treatment facilities or pre-military treatment facilities faces challenges because of the difficulty in availability, portability, storage, and shelf-life of platelets [32]. Platelets are highly limited in availability in battlefield settings and require special requirements of temperature, container, and additive solution. They are also at higher risk of bacterial contamination and can only stay for 5 to 7 days at room temperature. To improve the availability of platelets, Sen Gupta proposed SynthoPlate, a synthetic platelet, which could significantly reduce bleeding time and quickly stabilize blood pressure by enhancing the aggregation of activated platelets [33]. Dual-use (or application to both civilian and military uses) of this product in society at large may have a significant impact. This is because rural areas, more than an hour from a major medical center where blood supplies for transfusion are most available, probably cannot treat a major trauma case, and helicopter evacuation may be the only option.
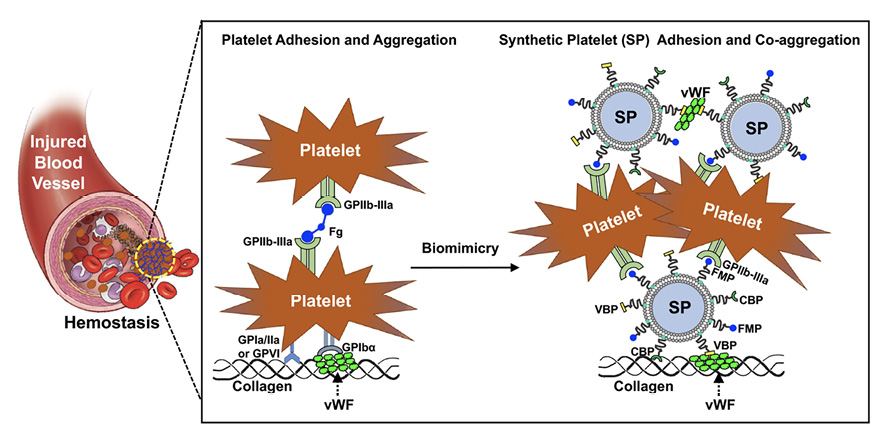
Figure 2. SynthoPlate mechanism of hemostatic activity. Platelets are cells that facilitate stoppage of bleeding (hemostasis) from injured blood vessels by forming a plug at the injury site via binding to specific proteins like Von Willebrand Factor (VWF) and collagen exposed at the site as well as by aggregating among each other via cross-linking of their surface integrin GPIIb-IIIa by fibrinogen (Fg). Synthetic Platelet (SP) systems are nanoparticles that mimic these platelet mechanisms and thus facilitate hemostasis by virtue of VWF-binding peptides (VBP), collagen-binding peptides (CBP), and Fg-mimetic peptides (FMP) combinatorially decorated on the particle surface.
Beyond specific mission readiness research pertinent to the needs of veterans and society at large, the DOD funds cancer research directed by Congressional mandates [34]. Since 1992, with the establishment of the Breast Cancer Research Program (BCRP), the DOD has allocated billions of dollars to cancer research programs, with a goal of improving the lives of service members and the civilian population [35]. These programs are focused on the most innovative types of treatment including a tagline of “Transforming Healthcare Through Innovation and Impactful Research.” In the cancer domain, invariably, this means treatments that lead to cures. Activating the host’s own immune system to cure cancer is one of the most promising approaches of the last decade [36].
A recent development has been cancer-targeting cells engineered from the patient’s own immune system, the T-cells, which, when re-injected in patients in a single dose, can cure many types of blood cancers [37]. These specially engineered T-cells, called chimeric antigen receptor T-cells or CAR-T, have shown the ability to cure patients by targeting a special signal called an epitope residing in the target cancer cells present in blood diseases like B-cell lymphoma or multiple myeloma [38]. The engineered CAR-T recognizes the cancer cell and its CD19 epitope signal and signals the host’s immune system to fight and, in many cases, even kill the cancer, providing long-lasting and durable protections for patients. However, if the cancer cell loses its epitope signal, it can evade the CAR-T, with recurrence of cancer [39]. A novel BAFF-CAR-T, which targets 3 sites unique to cancer cells, including a target called BAFF, which is under development in collaboration with Luminary Therapeutics to treat multiple myeloma and lymphoma patients [40].
Another cancer immune therapeutic approach that has also shown great promise is to target immune checkpoint proteins such as PD-1, PD-L1, and CTLA-4 [41]. These checkpoint proteins enable malignant cells to pass through the body undetected, by disabling T-cells. As a result, antagonists of these checkpoint proteins, known as immune checkpoint inhibitors, have recently begun to be recognized as the standard-of-care front-line treatment for increasing the efficacy of the body’s immune system in fighting certain cancers [42]. In contrast to these cell and drug-based approaches, Dr. Mei Zhang has identified a product from nature, a set of molecules easily purified from oats, that also activates the immune system and eradicates cancer in animal models [43]. Currently, the therapy is being tested in dog trials of a bone disease common in animals called osteosarcoma [44]. This is considered a good model for the human disease, and if it is proven efficacious in this animal model, seeking approval to test the idea in osteosarcoma patients would be pursued [45]. The potential for these immune-type therapies to impact health and disease is in its infancy, and these congressionally mandated funds, although small, may have an outsized impact on health research due to their translational and interdisciplinary focus. To date, most immune and cell therapy treatments are focused on cancer, but emerging evidence suggests they may be effective in mediating a host of inflammatory conditions as well, further amplifying the potential impact of these developments [46]. Thus, the DOD support for cancer therapies may revolutionize many health care challenges facing veterans and society at large.
Military medicine and medical research, supported by a combination of stakeholders including government, academia, hospitals, and industry, is a growing field that has significant overlap with society’s healthcare needs. Increasingly, the needs of warfighters, support personnel, veterans, and society overlap with biomedical research as a nexus of interest and source of potential solutions. Whether fighting the next outbreak with a novel vaccine or finding potential treatments to cure cancer, collaborations around military medicine, such as those seen in Cleveland and across Ohio, are translating novel discoveries towards the dual needs of warfighters and society. Further, the themes of human performance and resilience provide frameworks for developing effective societal responses to challenges.
We thank Ms. Anne DeChant and Dr. Ravenel Richardson for their efforts in coordinating the DOD-inspired symposium held at Case Western Reserve University. We thank Dr. Rohit Jain for leading biosensor civilian-use interviews, which allowed us to characterize the wider need for performance monitoring. We thank Springer Nature, original publisher of the image used in Figure 1, for open access use of the figure from Cho et al’s article in Biomaterials Research, under Creative Commons Attribution 4.0 International License.
This project was supported in part by the Clinical and Translational Science Collaborative of Northern Ohio, which is funded by the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Science Award grant, UM1TR004528. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
This material is based on research sponsored by the Air Force Research Laboratory under agreement number FA8650-18-2-5402. The US Government is authorized to reproduce and distribute reprints for government purposes notwithstanding any copyright notation thereon. The views and conclusions contained herein are those of the authors and should not be interpreted as necessarily representing the official policies or endorsements, either expressed or implied, of Air Force Research Laboratory (AFRL) or the US Government. ASG is supported by the National Institutes of Health (NIH) R01 award numbers HL121212, HL129179 and HL141080.ASG is also supported by the Department of Defense (DOD) contracts DM160354, PR191632, and PR211157. The content expressed in this manuscript is solely the responsibility of the author and does not necessarily represent the official views of the NIH and DOD. Distribution Statement A. Approved for public release: distribution is unlimited. AFRL-2023-2429.
MRC is a co-founder of Sensate Biosystems LLC, which is developing wearable sensors for performance monitoring. UAG has financial interests in Hemex Health Inc., BioChip Labs Inc., Xatec Inc., and DxNow Inc. These companies offer point-of-care diagnostics for hemoglobin disorders, anemia, and malaria; microfluidic biomarker assays for blood disorders; point-of-care global assays to evaluate the hemostatic process; microfluidic and bio-imaging technologies for IVF, forensics, and diagnostics. ASG is a co-founder, CTO, and Chair of Scientific Advisory Board for Haima Therapeutics LLC, a biotech company focused on the research and development of blood surrogate technologies.
1. Suran M. New Federal Agency for Biomedical and Health Research. JAMA. 2022;327(20):1949-. doi: 10.1001/jama.2022.8175.
2. Yeh KB, Du E, Olinger G, Boston D. Biotechnology and Biodefense Enterprise: An Industry Perspective on Defence Acquisition. Global Security: Health, Science and Policy. 2022;7(1):37-43. doi: 10.1080/23779497.2022.2102527.
3. DeChant AK, Fening S, Haag M, Harte W, Chance MR. Optimizing biomedical discoveries as an engine of culture change in an academic medical center. J Clin Transl Sci. 2022;6(1):e19. doi: 10.1017/cts.2021.888. PubMed PMID: 35291218; PMCID: PMC8889224.
4. Reizes O, Low M, Josyula VP, Ellis J, Vince DG. New programs for translating research to patient care: Lessons learned at the NIH Center for Accelerated Innovations at Cleveland Clinic. J Clin Transl Sci. 2021;5(1):e176. doi: 10.1017/cts.2021.849. PubMed PMID: 34849252; PMCID: PMC8596069.
5. Mackenbach JP. The rise and fall of diseases: reflections on the history of population health in Europe since ca. 1700. Eur J Epidemiol. 2021;36(12):1199-205. doi: 10.1007/s10654-021-00719-7. PubMed PMID: 33611677; PMCID: PMC7896827.
6. Ockenhouse CF, Magill A, Smith D, Milhous W. History of U.S. Military Contributions to the Study of Malaria. Military Medicine. 2005;170(suppl_4):12-6. doi: 10.7205/milmed.170.4s.12.
7. Ahmad W, Aquil Z, Alam S. Historical background of wound care. Hamdan Medical Journal. 2020;13(4):189-95. doi: 10.4103/hmj.Hmj_37_20.
8. Shah JB. The History of Wound Care. The Journal of the American College of Certified Wound Specialists. 2011;3(3):65-6. doi: 10.1016/j.jcws.2012.04.002.
9. Reilly RF. Medical and surgical care during the American Civil War, 1861-1865. Proc (Bayl Univ Med Cent). 2016;29(2):138-42. doi: 10.1080/08998280.2016.11929390. PubMed PMID: 27034545; PMCID: PMC4790547.
10. Figg L, Farrell-Beck J. Amputation in the Civil War: Physical and Social Dimensions. Journal of the History of Medicine and Allied Sciences. 1993;48(4):454-75. doi: 10.1093/jhmas/48.4.454.
11. Murray CK, Hinkle MK, Yun HC. History of Infections Associated With Combat-Related Injuries. Journal of Trauma and Acute Care Surgery. 2008;64(3):S221-S31. doi: 10.1097/TA.0b013e318163c40b. PubMed PMID: 00005373-200803001-00004.
12. King B, Jatoi I. The mobile Army surgical hospital (MASH): a military and surgical legacy. J Natl Med Assoc. 2005;97(5):648-56. PubMed PMID: 15926641; PMCID: PMC2569328.
13. Blyth DM, Yun HC, Tribble DR, Murray CK. Lessons of war: Combat-related injury infections during the Vietnam War and Operation Iraqi and Enduring Freedom. J Trauma Acute Care Surg. 2015;79(4 Suppl 2):S227-35. doi: 10.1097/ta.0000000000000768. PubMed PMID: 26406435; PMCID: PMC4586048.
14. Chen Y, Chau J, Yoon J, Hladky J. Rapid, label-free pathogen identification system for multidrug-resistant bacterial wound infection detection on military members in the battlefield. PLOS ONE. 2022;17(5):e0267945. doi: 10.1371/journal.pone.0267945.
15. Gajda E, Krzowski Ł, Kowalczuk K, Pabin A, Maculewicz E. Influence of mosquito-borne biological agents on health risks among soldiers and military personnel. Annals of Agricultural and Environmental Medicine. 2023;30(1):2-8. doi: 10.26444/aaem/155003.
16. Tribble DR, Murray CK, Lloyd BA, Ganesan A, Mende K, Blyth DM, Petfield JL, McDonald J. After the Battlefield: Infectious Complications among Wounded Warriors in the Trauma Infectious Disease Outcomes Study. Military Medicine. 2019;184(Supplement_2):18-25. doi: 10.1093/milmed/usz027.
17. Casadevall A. Climate change brings the specter of new infectious diseases. The Journal of Clinical Investigation. 2020;130(2):553-5. doi: 10.1172/JCI135003.
18. Carlson CJ, Albery GF, Merow C, Trisos CH, Zipfel CM, Eskew EA, Olival KJ, Ross N, Bansal S. Climate change increases cross-species viral transmission risk. Nature. 2022;607(7919):555-62. doi: 10.1038/s41586-022-04788-w.
19. Gupta S, Rouse BT, Sarangi PP. Did Climate Change Influence the Emergence, Transmission, and Expression of the COVID-19 Pandemic? Front Med (Lausanne). 2021;8:769208. doi: 10.3389/fmed.2021.769208. PubMed PMID: 34957147; PMCID: PMC8694059.
20. Damato EG, Fillioe SJ, Margevicius SP, Mayes RS, Somogyi JE, Vannix IS, Abdollahifar A, Turner AM, Ilcus LS, Decker MJ. Increased Serum Levels of Proinflammatory Cytokines Are Accompanied by Fatigue in Military T-6A Texan II Instructor Pilots. Frontiers in Physiology. 2022;13. doi: 10.3389/fphys.2022.876750.
21. Singh S, Anshita D, Ravichandiran V. MCP-1: Function, regulation, and involvement in disease. International Immunopharmacology. 2021;101:107598. doi: 10.1016/j.intimp.2021.107598.
22. Zhang J, Chen M, Peng Y, Li S, Han D, Ren S, Qin K, Li S, Han T, Wang Y, Gao Z. Wearable biosensors for human fatigue diagnosis: A review. Bioengineering & Translational Medicine. 2023;8(1):e10318. doi: 10.1002/btm2.10318.
23. Madhurantakam S, Muthukumar S, Prasad S. Emerging Electrochemical Biosensing Trends for Rapid Diagnosis of COVID-19 Biomarkers as Point-of-Care Platforms: A Critical Review. ACS Omega. 2022;7(15):12467-73. doi: 10.1021/acsomega.2c00638.
24. Arishi WA, Alhadrami HA, Zourob M. Techniques for the Detection of Sickle Cell Disease: A Review. Micromachines. 2021;12(5):519. PubMed PMID: doi: 10.3390/mi12050519.
25. Heidt B, Siqueira WF, Eersels K, Diliën H, van Grinsven B, Fujiwara RT, Cleij TJ. Point of Care Diagnostics in Resource-Limited Settings: A Review of the Present and Future of PoC in Its Most Needed Environment. Biosensors. 2020;10(10):133. PubMed PMID: doi: 10.3390/bios10100133.
26. Jain R FE, Kiselar J, Lodowski DT, Chance MR, editor. A multipronged footprinting map validates the predicted structure of stress biomarker Neuropeptide Y (NPY) and its binding with novel peptide binders. [Conference Abstract]. Annual Symposium of The Protein Society; 2023 https://www.cell.com/biophysj/pdf/S0006-3495(22)03446-4.pdf; San Diego, California: Biophysical Journal.
27. Radwan O, Brothers MC, Coyle V, Chapleau ME, Chapleau RR, Kim SS, Ruiz ON. Electrochemical biosensor for rapid detection of fungal contamination in fuel systems. Biosensors and Bioelectronics. 2022;211:114374. doi: 10.1016/j.bios.2022.114374.
28. Kinnamon D, Ghanta R, Lin K-C, Muthukumar S, Prasad S. Portable biosensor for monitoring cortisol in low-volume perspired human sweat. Scientific Reports. 2017;7(1):13312. doi: 10.1038/s41598-017-13684-7.
29. Cho I-H, Kim DH, Park S. Electrochemical biosensors: perspective on functional nanomaterials for on-site analysis. Biomaterials Research. 2020;24(1):6. doi: 10.1186/s40824-019-0181-y.
30. Moore EE, Moore HB, Kornblith LZ, Neal MD, Hoffman M, Mutch NJ, Schöchl H, Hunt BJ, Sauaia A. Trauma-induced coagulopathy. Nature Reviews Disease Primers. 2021;7(1):30. doi: 10.1038/s41572-021-00264-3.
31. Spinella PC, Dunne J, Beilman GJ, O’Connell RJ, Borgman MA, Cap AP, Rentas F. Constant challenges and evolution of US military transfusion medicine and blood operations in combat. Transfusion. 2012;52(5):1146-53. doi: 10.1111/j.1537-2995.2012.03594.x. PubMed PMID: 22575063.
32. Stubbs JR, Homer MJ, Silverman T, Cap AP. The current state of the platelet supply in the US and proposed options to decrease the risk of critical shortages. Transfusion. 2021;61(1):303-12. doi: 10.1111/trf.16140.
33. Hickman DA, Pawlowski CL, Shevitz A, Luc NF, Kim A, Girish A, Marks J, Ganjoo S, Huang S, Niedoba E, Sekhon UDS, Sun M, Dyer M, Neal MD, Kashyap VS, Sen Gupta A. Intravenous synthetic platelet (SynthoPlate) nanoconstructs reduce bleeding and improve ‘golden hour’ survival in a porcine model of traumatic arterial hemorrhage. Scientific Reports. 2018;8(1):3118. doi: 10.1038/s41598-018-21384-z.
34. Young-McCaughan S, Rich IM, Lindsay GC, Bertram KA. The Department of Defense Congressionally Directed Medical Research Program: innovations in the federal funding of biomedical research. Clin Cancer Res. 2002;8(4):957-62. PubMed PMID: 11948100.
35. Lidie KB, Green Parker MC, Martinelli AM, Rowe SS, Leggit JC. Making an Impact: Congressionally Directed Medical Research Programs Complement Other Sources of Biomedical Funding. Fed Pract. 2015;32(1):20-7. PubMed PMID: 30766019; PMCID: PMC6363290.
36. Liu X, Li Y, Sun X, Muftuoglu Y, Wang B, Yu T, Hu Y, Ma L, Xiang M, Guo G, You C, Gao X, Wei Y. Powerful anti-colon cancer effect of modified nanoparticle-mediated IL-15 immunogene therapy through activation of the host immune system. Theranostics. 2018;8(13):3490-503. doi: 10.7150/thno.24157.
37. Wong DP, Roy NK, Zhang K, Anukanth A, Asthana A, Shirkey-Son NJ, Dunmire S, Jones BJ, Lahr WS, Webber BR, Moriarity BS, Caimi P, Parameswaran R. A BAFF ligand-based CAR-T cell targeting three receptors and multiple B cell cancers. Nature Communications. 2022;13(1):217. doi: 10.1038/s41467-021-27853-w.
38. Kochenderfer JN, Rosenberg SA. Treating B-cell cancer with T cells expressing anti-CD19 chimeric antigen receptors. Nature Reviews Clinical Oncology. 2013;10(5):267-76. doi: 10.1038/nrclinonc.2013.46.
39. Qiu S, Pan Y, Shi S, Omotoyosi FF, Chen K, Guo Z, Lü P. Genetic Mechanism of Leukemia Relapse Following CD19 Chimeric Antigen Receptor T Cell Therapy. Cancer Biotherapy and Radiopharmaceuticals. 2021;37(5):335-41. doi: 10.1089/cbr.2020.4630.
40. Parameswaran R, Wong D, Zhang K, Asthana A, de Lima M, Caimi PF. Ligand Based CAR T-Cell Targeting BAFF Receptors Asa Novel Therapy for B Cell Malignancies. Blood. 2020;136(Supplement 1):31-2. doi: 10.1182/blood-2020-141009.
41. Pandey P, Khan F, Qari HA, Upadhyay TK, Alkhateeb AF, Oves M. Revolutionization in Cancer Therapeutics via Targeting Major Immune Checkpoints PD-1, PD-L1 and CTLA-4. Pharmaceuticals. 2022;15(3):335. PubMed PMID: doi: 10.3390/ph15030335.
42. Szabados B, Prendergast A, Jackson-Spence F, Choy J, Powles T. Immune Checkpoint Inhibitors in Front-line Therapy for Urothelial Cancer. European Urology Oncology. 2021;4(6):943-7. doi: 10.1016/j.euo.2021.02.010.
43. Zhang M, Chun L, Sandoval V, Graor H, Myers J, Nthale J, Rauhe P, Senders Z, Choong K, Huang AY, Kim J. Systemic administration of β-glucan of 200 kDa modulates melanoma microenvironment and suppresses metastatic cancer. Oncoimmunology. 2018;7(2):e1387347. doi: 10.1080/2162402x.2017.1387347. PubMed PMID: 29308312; PMCID: PMC5749667.
44. Makielski KM, Mills LJ, Sarver AL, Henson MS, Spector LG, Naik S, Modiano JF. Risk Factors for Development of Canine and Human Osteosarcoma: A Comparative Review. Veterinary Sciences. 2019;6(2):48. PubMed PMID: doi: 10.3390/vetsci6020048.
45. Mason NJ. Comparative Immunology and Immunotherapy of Canine Osteosarcoma. In: Kleinerman ES, Gorlick R, editors. Current Advances in the Science of Osteosarcoma: Research Perspectives: Tumor Biology, Organ Microenvironment, Potential New Therapeutic Targets, and Canine Models. Cham: Springer International Publishing; 2020. p. 199-221.
46. Regmi S, Pathak S, Kim JO, Yong CS, Jeong J-H. Mesenchymal stem cell therapy for the treatment of inflammatory diseases: Challenges, opportunities, and future perspectives. European Journal of Cell Biology. 2019;98(5):151041. doi: 10.1016/j.ejcb.2019.04.002.
Submitted May 23, 2023 | Accepted July 7, 2023 | Published September 8, 2023
Copyright © 2023 The Author(s). This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License.