
Owen Jensen1,2, Shubhanshi Trivedi1, Kelin Li3, Jeffrey Aubé3, J. Scott Hale2, Edward T. Ryan4,5,6, Daniel T. Leung1, 2*
1 Division of Infectious Diseases, Department of Internal Medicine, University of Utah School of Medicine, Salt Lake City, Utah
2 Division of Microbiology & Immunology, Department of Pathology, University of Utah School of Medicine, Salt Lake City, Utah
3 Division of Chemical Biology and Medicinal Chemistry, UNC Eshelman School of Pharmacy, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina
4 Division of Infectious Disease, Massachusetts General Hospital, Boston, Massachusetts
5 Department of Medicine, Harvard Medical School, Boston, Massachusetts
6 Department of Immunology and Infectious diseases, Harvard School of Public Health, Boston, Massachusetts
Daniel T. Leung
E-mail: daniel.leung@utah.edu
Jensen O, Trivedi S, Li K, Aubé J, Hale JS, Ryan ET, Leung DT, Use of a MAIT Activating Ligand, 5-OP-RU, as a Mucosal Adjuvant in a Murine Model of Vibrio cholerae O1 Vaccination. Pathogens and Immunity. 2022;7(1): 122–144. doi: 10.20411/pai.v7i1.525.
10.20411/pai.v7i1.525
Background: Mucosal-associated invariant T (MAIT) cells are innate-like T cells enriched in the mucosa with capacity for B-cell help. We hypothesize that targeting MAIT cells, using a MAIT-activating ligand as an adjuvant, could improve mucosal vaccine responses to bacterial pathogens such as Vibrio cholerae.
Methods: We utilized murine models of V. cholerae vaccination to test the adjuvant potential of the MAIT-activating ligand, 5-(2-oxopropylideneamino)-6-D-ribitylaminouracil (5-OP-RU). We measured V. cholerae-specific antibody and antibody-secreting cell responses and used flow cytometry to examine MAIT-cell and B-cell phenotype, in blood, bronchoalveolar lavage fluid (BALF), and mucosal tissues, following intranasal vaccination with live V. cholerae O1 or a V. cholerae O1 polysaccharide conjugate vaccine.
Results: We report significant expansion of MAIT cells in the lungs (P < 0.001) and BALF (P < 0.001) of 5-OP-RU treated mice, and higher mucosal (BALF, P = 0.045) but not systemic (serum, P = 0.21) V. cholerae O-specific-polysaccharide IgG responses in our conjugate vaccine model when adjuvanted with low-dose 5-OP-RU. In contrast, despite significant MAIT cell expansion, no significant differences in V. cholerae-specific humoral responses were found in our live V. cholerae vaccination model.
Conclusions: Using a murine model, we demonstrate the potential, as well as the limitations, of targeting MAIT cells to improve antibody responses to mucosal cholera vaccines. Our study highlights the need for future research optimizing MAIT-cell targeting for improving mucosal vaccines.
Keywords: Cholera; MAIT cells; cholera vaccines; mucosal adjuvant; 5-OP-RU; conjugate vaccine
Mucosal-associated invariant T (MAIT) cells are innate-like T cells that display a semi-invariant T-cell receptor (TCR) primarily composed of the TCRα chain, Vα7.2 linked to Jα33/12/20 in humans or Vα19 linked to Jα33 in mice, with a limited array of TCR β chains [1–3]. The MAIT TCR recognizes pyrimidine intermediates from the riboflavin synthesis pathway presented on the evolutionarily conserved major histocompatibility complex (MHC) Class I-related protein, MR1 [1, 4–6]. MAIT cells can be activated via TCR:MR1 engagement or through cytokine stimulation, and were initially appreciated for their potent production of pro-inflammatory cytokines, including interferon-γ (IFNγ), tumor necrosis factor-α (TNFα), interleukin-17A (IL-17A), and cytotoxic molecules [7–9]. Recent studies have shown associations between MAIT cells and adaptive immune responses, including the ability for MAIT cells to provide B-cell help in vitro and in vivo, suggesting that MAIT cells might be potential targets for improving responses to mucosal vaccines, including against mucosal pathogens [10–18]. Pertinent to this, we and others have previously shown that MAIT cell frequency and activation are associated with LPS-specific antibody responses in the blood of cholera patients [10] and Shigella vaccines [11]. We have further shown that human MAIT cells are able to promote B-cell differentiation and antibody production in vitro [12], and that T-follicular helper (Tfh)-like MAIT cells in tonsils can produce key B-cell help cytokines, such as IL-21 [17]. MAIT cells have also been shown to promote antibody production in a mouse model of lupus [13], a macaque model of SIV vaccination [14], and are correlated with peptide specific T-cell responses in humans given adenovirus vector [16] and SARS-CoV2 mRNA vaccines [18]. Based on these data, we hypothesized that MAIT cells may be promising targets for mucosal vaccine adjuvants to improve adaptive B- and T-cell responses.
Cholera is a mucosal infection of global importance. Cholera is an acute dehydrating diarrheal disease caused by the non-invasive bacterial pathogen, Vibrio cholerae, which causes 2-5 million cases per year resulting in tens of thousands of deaths [19]. Efforts to control cholera globally include the administration of oral cholera vaccines (OCVs) [20]. OCVs likely provide protection via the development of antibodies against V. cholerae lipopolysaccharide (LPS) [21]. Despite increased use in endemic areas, OCV protection remains limited with an average 2-dose efficacy of 58% in adults and 30% in children under 5 years of age [22]. Notably, V. cholerae LPS memory B-cell and antibody responses highly correlate with cholera protection [23, 24], and these responses are markedly reduced in children under 5 years of age receiving OCVs compared to children with confirmed cholera infection [25]. This reduced ability to mount effective polysaccharide-specific antibody responses to vaccines is consistent with previous reports of reduced bacterial polysaccharide antibody in infants in response to polysaccharide vaccines [26]. Thus, the need for improved vaccines to enhance anti-V. cholerae polysaccharide immune responses in children is needed.
Mucosal adjuvants have the potential to improve mucosal vaccine efficacy through many mechanisms, including enhancing vaccine delivery, reducing tolerogenic responses, and increasing mucosal immune cell-specific targeting to improve antigen presentation and the development of adaptive immune responses [27]. In particular, using adjuvants to target innate-like lymphocytes such as invariant natural killer T (iNKT), γδ T, and MAIT cells holds potential to improve mucosal vaccines. Targeting iNKT cells using iNKT ligands, α-galactosylceramide (α-GC) and α-GC analogs, has been studied extensively in mouse vaccination models with varying success [28]. Notably, oral α-GC gavage along with OCV in mice resulted in higher anti-LPS specific immune responses compared to OCV alone [29]. Despite these promising results, efficacy in humans remains uncertain as iNKT frequency in human intestines is approximately 10-fold lower than in mice [30–32]. Alternatively, MAIT cells are enriched in human blood, liver, and mucosa [7, 33–37] and thus may be promising targets for OCV adjuvants.
The recent discovery of MR1 binding and MAIT-activating pyrimidine, 5-OP-RU, has allowed for improved MAIT identification via MR1-5-OP-RU tetramer binding and direct MAIT targeting in vitro and in vivo [38–45]. Until bound by MR1, 5-OP-RU is highly unstable and is formed by the reaction of an early vitamin B synthesis intermediate, 5-amino-6-D-ribitylaminouracil (5-A-RU), and methylglyoxal (MGO) [38]. When administered intranasally with toll-like receptor (TLR) agonists in mice, 5-OP-RU significantly expands MAIT cells and has been shown to improve protection in mouse models of mucosal Salmonella, Legionella, and Mycobacterium spp. challenge [39, 40, 42]. Recently, a more stable synthetic preparation of 5-A-RU has been developed allowing for easier storage and administration of the MAIT ligand [46]. Utilizing this tool as a mucosal vaccine adjuvant, we aimed to determine if activating MAIT cells in the presence of V. cholerae antigens is a viable option to improve humoral immune responses; we used live V. cholerae and V. cholerae polysaccharide conjugate antigens in murine vaccination models to address this issue. We report modest effects on mucosal immune responses in mice treated with a V. cholerae polysaccharide conjugate vaccine along with MAIT ligand, although there were no significant differences in humoral responses in our live V. cholerae vaccination model. Our study, by exhibiting both the potential and limitation of targeting MAIT ligands as adjuvants, adds to the growing body of work investigating MAIT ligands in the context of prophylactic and therapeutic vaccines.
Six- to eight-week-old WT C57BL/6J female mice were acquired from the Jackson Laboratory (Bar Harbor, USA) before the start of the experiment. All mice were housed under specific pathogen-free conditions and all animal experiments were performed under strict accordance with the National Institutes of Health (NIH) Guide for Care and Use of Laboratory Animals and institutional guidelines for animal care at the University of Utah under approved protocol no. 19-08017.
The following intranasal challenge model was adapted from Nygren et al [47] and Vorkas et al [42], to induce systemic and mucosal V. cholerae-specific antibody responses and MAIT cell expansion. In brief, lightly anesthetized mice were vaccinated intranasally on day 0 with 106 colony forming units (CFU) live V. cholerae O1 Inaba strain N16961 with PBS, or 16.67 mg Pam2CSK4 (Pam2) (Invitrogen), or 16.67 µg Pam2CSK4 plus 200 nmol 5-A-RU (as described in [46]) and 50 µM MGO (Sigma-Aldrich) in 100 µL (50 µL per nares). On day 1, 2, and 4, mice were subsequently given either intranasal PBS or 200 nmol 5-A-RU plus 50mM MGO. All mice were boosted with 106 live V. cholerae O1 Inaba on day 28. For 7 days following live V. cholerae inoculations, mice were weighed daily and monitored for signs of clinical pneumonia symptoms. Mice that lost >20% of their initial body weight were euthanized. Blood samples were collected weekly by submandibular bleeds and serum was isolated using BD microtainer SST tubes (BD Biosciences). On day 35 final blood samples were collected and then mice were euthanized using isoflurane. Bronchoalveolar lavage fluid (BALF) was collected by exposing the trachea of the euthanized mouse and, using a scalpel, cutting a small hole for catheter (23G needle inserted into polyethylene tubing) insertion. The catheter was then stabilized by tying a knot with surgical silk, and a 1 mL syringe with sterile PBS was attached to the catheter needle. PBS was then gently injected into the lungs and slowly aspirated. This process was repeated with 1 mL of PBS for a total of 2 mL of BALF collected. BALF was centrifuged at 400g x 5 minutes and supernatant was collected and frozen at -80°C for enzyme-linked immunosorbent assay (ELISA). BAL cells were resuspended in FACS buffer for flow cytometry. Lungs were then perfused with 5 mL cold PBS, and single cell suspensions were prepared using the gentleMACS Lung Dissociation Kit (Miltenyi Biotech) according to the manufacturer’s protocol. Mediastinal lymph nodes (MLN) and spleen were then excised and ground through a 70 µm filter. Lungs and spleens were then treated with ACK lysis buffer (Thermo Fisher Scientific) to lyse red blood cells. Single cell suspensions were used for flow cytometry analysis.
The following V. cholerae OSP plus MAIT ligand vaccination model was adapted from Pankhurst et al [48]. On day 0, 14, and 28, mice were lightly anesthetized and vaccinated intranasally with 20 µg V. cholerae O1 Ogawa strain PIC158 OSP:BSA (Courtesy of the Edward Ryan lab, Harvard University, Boston) and 50 µM MGO plus PBS or 75 nmol 5-A-RU in 50 µL (25 µL per nares). OSP:BSA was prepared as described [49]. Serum samples were collected as described above on day 7, 21, and 35. Mice were euthanized on day 35, and BALF, lungs, and MLN were collected as described above.
Flow cytometry was performed using standard cell-surface staining techniques using directly conjugated fluorochrome antibodies and analyzed on the Cytek Aurora (Cytek Biosciences). All analysis was performed using FlowJo version 10.8.0 (BD Biosciences). Prior to surface staining, tissue single-cell suspensions were incubated in Fixable Viability Dye eFluor 780 (eBioscience) to exclude dead cells, washed, and then incubated in anti-mouse CD16/CD32 Fc block (Biolegend) and unlabeled MR1-6-formylpterin-tetramer (6-FP) (NIH Tetramer core) to reduce non-specific binding. Cells were then incubated for 20 minutes at room temperature with mouse PE conjugated 5-OP-RU MR1-tetramer (NIH Tetramer core) and the following antibodies: anti-CD4-FITC/APC-Fire810 (clone GK1.5, Tonbo Biosciences/Biolegend), anti-CD8α-AF700 (clone 53-6.7, Biolegend), anti-TCRβ-BUV496/BV421 (clone H57-597, BD Biosciences/Biolegend), anti-CD3-BUV395 (clone 17A2, Biolegend), anti-CD69-BV510 (clone H1.2F3 Biolegend), anti-B220-PE-Cy5 (clone RA3-6B2, Biolegend), anti-CD19-BV711 (clone 6D5, Biolegend), anti-CD44-BV650 (clone IM7, Biolegend), anti-CD38-PE-Cy7 (clone 90, Biolegend), anti-IgD-BV510 (clone 11-26c.2a, Biolegend), anti-CD27-FitC (clone LG.3A10, Biolegend), anti-CD138-BV605 (clone 281-2, Biolegend).
Serum and BALF OSP:BSA, cholera toxin (CT), and V. cholerae-specific lysate IgM, IgG, and IgA ELISAs were performed as described in Jensen et al [17]. V. cholerae O1 Inaba and Ogawa specific OSP:BSA were used for the live V. cholerae and OSP:BSA cholera vaccination model ELISAs, respectively. For all ELISAs, serum was diluted 1:20, and BALF samples were diluted 1:2. IL-21 concentration from BALF and lung homogenate was measured using a DuoSet Mouse IL-21 ELISA kit (RnD Systems).
For OSP IgG and IgA ELISpots, 96-well MultiScreen-HA filter plates (Sigma) were coated with 0.1 μg/well with V. cholerae O1 Inaba OSP:BSA in PBS and incubated overnight at 4°C. Cholera toxin ELISpots were first coated overnight with 0.1 μg/well monosialoganglioside GM1 (Sigma) in carbonate buffer overnight at 4°C, washed 3X with 0.05% Tween20 in PBS (PBS-T) and then incubated overnight at 4°C with 0.25 μg/well CT (Sigma). OSP and CT ELISAs were then blocked with warm 200 μL/well of R10 media (RPMI + L-Glutamate (Gibco), 10% fetal bovine serum (FBS) + 1X Penn/Strep (Thermo Fisher Scientific)) for 2 hours at 37°C. Then, R10 was removed by decanting and single-cell suspensions of lung and spleen cells, resuspended in R10, were added at dilutions of 1x105 and 1x106 cells/well and incubated at 37°C for 5 hours. Plates were washed 3X with PBS and 3X with PBS-T, and then anti-mouse IgG/IgA-biotin (Southern Biotech) diluted 1:1000 in PBS-T was added and incubated overnight at 4°C. Plates were then washed 3X with PBS-T and incubated for 1 hour at room temperature in the dark with horseradish peroxidase-Avidin conjugate (Avidin-HRP, eBioscience). Following wash, plates were developed with AEC (3 amino-9-ethyl-carbozole) and enumerated compared to negative control wells.
All statistical comparisons were made using 2-tailed Mann-Whitney U tests using Prism version 9.2.0 (GraphPad Software, La Jolla, CA). All graphs were also created using Prism.
To study the mucosal adjuvant capacity of the MAIT activating ligand, 5-OP-RU, we utilized an intranasal prime-boost vaccination model with live V. cholerae, previously shown by our group [17] and others [47] to induce mucosal and systemic V. cholerae-specific LPS and CT antibody responses in wildtype (WT) C57Bl/6 (B6) mice. B6 mice were inoculated on day 0 and day 28 with 1x106 CFU live V. cholerae O1 Inaba along with either toll-like receptor (TLR) 2/6 agonist, Pam2CSK4 (Pam2) alone, or in combination with 4 doses of 5-A-RU and MGO as outlined in Figure 1A. At day 35 (7 days post boost) mice were euthanized and we analyzed the frequency and activation of MAIT cells in lungs, BALF, mediastinal lymph nodes (MLN), and spleens by flow cytometry. We defined MAIT cells as live CD19- CD3+ CD44High TCRβ+ MR1-tetramer+ (Supplementary Figure 1A) and measured MAIT-cell activation based on expression of CD69. In line with previous reports, we found significantly higher MAIT cell frequency in the lungs of 5-A-RU treated mice (median=2.6%) compared to the PBS (V. cholerae only) (median=0.08%), and Pam2 (V. cholerae + Pam2) (median=0.15%) groups (Figure 1B and C). MAIT cell frequency was also higher in BALF (Figure 1D), while only moderately higher in the MLN and spleen (Figure 1E and F). Frequencies of non-MAIT CD8 and CD4 T cells were also higher in the lungs and BALF of 5-A-RU treated mice compared to the PBS group, though this is likely largely driven by TLR2/6 stimulation as the 5-A-RU group was not statistically different than the Pam2 group alone (Supplementary Figure 1B-E). Furthermore, despite higher MAIT-cell frequency in lungs and BALF, we found no differences in CD69 expression frequency in any tissue at the experiment endpoint (Figure 1G-J).
Given that our previous demonstration of the sufficiency of MAIT cells to promote V. cholerae-specific antibody responses, and the associations of MAIT cells with LPS-specific antibody responses following human V. cholerae O1 infection [10] and Shigella vaccination [11], we hypothesized that MAIT expansion in the mouse mucosa may lead to increased LPS antibody responses. To this end, we collected weekly serum samples and BALF at endpoint and measured IgG, IgA, and IgM antibody responses against CT (protein antigen), V. cholerae LPS O-specific polysaccharide (OSP) (polysaccharide antigen), and total V. cholerae lysate (Figure 2 and Supplementary Figure 2). CT and OSP are the immunodominant antigens following V. cholerae O1 infection [21], and OSP responses are associated with protection against subsequent V. cholerae O1 infection [50, 51]. In concurrence with our previous work [17], the PBS-adjuvanted group (receiving only V. cholerae) induced robust serum CT-IgG, CT-IgA, and OSP-IgG responses, although variable OSP-IgA responses (Figure 2A-D). In addition to systemic antibody responses, we also recorded strong mucosal BALF CT and OSP antibody responses (Figure 2E-H). We did not find any significant differences between either the Pam2 or the 5-A-RU group, compared to the PBS group, in any systemic or mucosal CT- or OSP-specific antibody responses at any days. We found non-significantly higher BALF OSP-IgG (P=0.212) and IgA (P=0.200) in the 5-A-RU groups (Figure 2G and H). Additionally, no statistically significant differences were found in any systemic or mucosal CT-IgM, OSP-IgM or V. cholerae-lysate-specific antibody responses as determined by ELISA (Supplementary Figure 2).

Figure 1. MAIT cells expand and persist in lungs and bronchoalveolar lavage fluid (BALF) following intranasal 5-A-RU treatment. (A) Live intranasal MAIT ligand plus V. cholerae O1 Inaba vaccination model timeline. (B) Representative FACS plots and (C-F) frequency as a percentage of CD3+ cells of MAIT cells (TCRβ+MR1-Tetramer+) from (C) lung, (D) BALF, (E) MLN, and (F) spleen gated on Live CD3+ CD19- CD44+ cells. (G-J) Frequency of MAIT cells expressing CD69 in (G) lung, (H) BALF, (I) MLN, and (J) spleen. Data are represented as Median with IQR from 3 independent experiments. n=11-12 mice per group. *P< 0.05, **P > 0.01, ***P > 0.001, ***P > 0.0001 by 2-tailed Mann-Whitney U test.

Figure 2. Intranasal 5-A-RU has no effect on protein or polysaccharide-specific antibody responses when administered with a live V. cholerae vaccination. (A-D) Serum day 35 endpoint (left) and time-course (right) (A) CT-IgG, (B) CT-IgA, (C) OSP-IgG, and (D) OSP-IgA ELISAs. (E-H) BAL fluid day 35 endpoint (E) CT-IgG, (F) CT-IgA, (G) OSP-IgG, and (H) OSP-IgA ELISAs. Data are represented as ELISA units measured kinetically and normalized to positive control pooled serum from WT B6 mice intranasally vaccinated with live V. cholerae. Data are represented as Median with IQR from 3 independent experiments. n=11-12 mice per group. *P < 0.05, **P > 0.01, ***P > 0.001, ***P > 0.0001 by 2-tailed Mann-Whitney U test.
In addition to antibody responses, we also examined the development of IgG and IgA OSP and CT specific antibody-secreting cells (ASC) in both the mucosa (lungs) and secondary lymphoid organs (spleen) using ELISpots following intranasal 5-A-RU treatment. We found no significant differences in CT-IgG (Figure 3A-C) or CT-IgA (Figure 3A, D, and E) ASCs in lungs or spleen between 5-A-RU and Pam2 or PBS groups. Despite clear OSP-IgG antibodies in both serum (Figure 2C) and BALF (Figure 2G), we found very few (<15 ASC per 1x106 cells) OSP-IgG ASCs in both lungs and spleen of all groups (Figure 3F-H). OSP-IgA ASCs were significantly higher in the lungs of the 5-A-RU group compared to the PBS group (P=0.042), although were similar to the Pam2 group (Figure 3F and I). Similarly, we report non-statistically significant higher splenic OSP-IgA
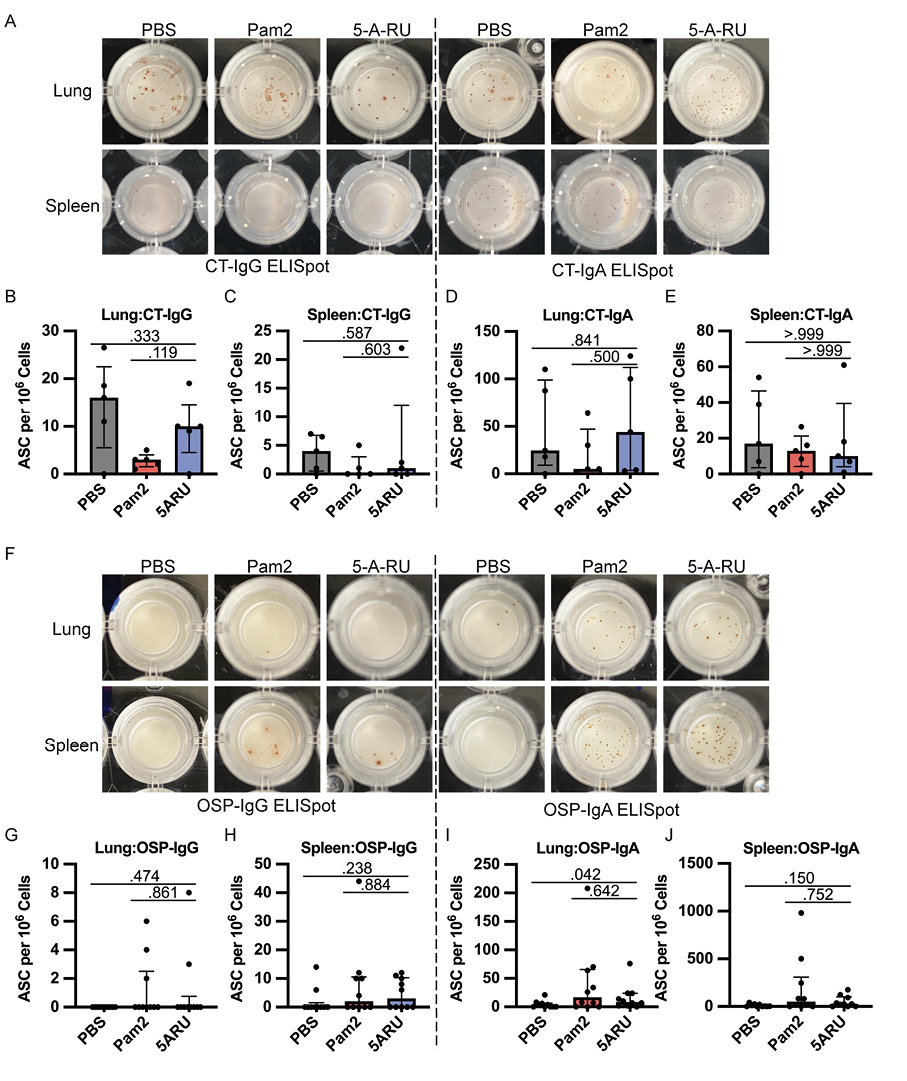
Figure 3. Intranasal 5-A-RU has no effect on protein or polysaccharide-specific antibody-secreting cell frequency in live V. cholerae vaccination. (A) Representative CT-IgG (left) and CT-IgA (right) ELISpot images from day 35 endpoint lung (top) and spleen (bottom) cells. (B-E) lung and spleen CT-IgG (B-C) and CT-IgA (D-E) ASC frequency per 106 live cells. Data are represented as Median with IQR from 1 independent experiment. n=5 mice per group. (F) Representative OSP-IgG (left) and OSP-IgA (right) ELISpot images from day 35 endpoint lung (top) and spleen (bottom) cells. (G-J) lung and spleen OSP-IgG (G-H) and OSP-IgA (I-J) ASC frequency per 106 live cells. Data are represented as Median with IQR from 2 independent experiments. n=10 mice per group. P values determined by 2-tailed Mann-Whitney U test.
ASCs in the 5-A-RU group compared to the PBS group (P=0.150). Overall, despite substantial expansion and persistence of MAIT cells in both the mucosa and secondary lymphoid organs following 5-A-RU treatment, we found little difference in humoral pathogen-specific immune responses.
Due to the relatively robust anti-CT and OSP responses following the live V. cholerae intranasal challenge (Figures 1-3), we hypothesized that any potential adjuvant impact of intranasal 5-A-RU on V. cholerae-specific antibody responses may be undetectable due to a ceiling effect. In support of this hypothesis, a recent study by Pankhurst et al [48] demonstrated that low-dose 5-A-RU augmented influenza hemagglutinin (HA) mucosal and systemic antibody responses. Thus, we sought to determine if a low-dose 5-A-RU treatment without added TLR agonists would enhance B-cell responses to an intranasal vaccination of V. cholerae O1 Ogawa OSP conjugated to bovine serum albumin (OSP:BSA). We inoculated mice on day 0, 14, and 28 with 20 mg of V. cholerae Ogawa OSP:BSA and 100 nmol MGO +/- 75 nmol 5-A-RU. Serum samples were collected on day 7 and 21, and mice were euthanized, and tissue harvested on day 35 (Figure 4A). To confirm that low dose 5-A-RU treatment without TLR agonists was effective at targeting MAIT cells, we analyzed MAIT and non-MAIT T cell frequency in the lungs, BALF, and MLN using flow cytometry. MAIT cells were defined as live B220- TCRβ+ CD44High MR1-tetramer+ cells (Supplementary Figure 3A). We found significantly higher MAIT frequency in the lungs (P<0.001) and BALF (P<0.001) of 5-A-RU treated mice compared to MGO alone comprising a median of 6.6% and 10% of total TCRβ+ T cells respectively (Figure 4B-D). In contrast, MAIT cell frequency in MLNs remained unchanged between MGO and 5-A-RU groups (Figure 4B and E). No significant differences between groups were found in frequencies of non-MAIT CD8 and CD4 T cells in all tissues (Figure 4C-E).
After establishing that low dose 5-A-RU expands MAIT cells in the mucosa, we next sought to test whether co-administration of 5-A-RU with V. cholerae OSP:BSA would enhance anti-OSP-specific (polysaccharide) antibody responses. To test this hypothesis, we examined OSP-specific antibody responses in serum and BALF following OSP intranasal immunization with or without 5-A-RU adjuvant. We report no differences in serum OSP-IgM antibody responses between MGO and 5-A-RU (Supplementary Figure 4A), and non-statistically significant higher OSP-IgG (P=0.210; Figure 5A and B). Notably, we saw statistically significant (P=0.045) higher BALF OSP-IgG antibodies in the 5-A-RU relative to the MGO alone group (Figure 5C). Despite the mucosal nature of the infection, serum and BALF OSP-IgA (Supplementary Figure 4C and D) and OSP-IgM (Supplementary Figure 4A and B) responses in almost all mice were all below the ELISA LOD (dotted line). To investigate the relationship between OSP antibody response and MAIT cell frequency in BALF and lungs (Figure 4B-D) we used simple linear regression analysis. Analyzing only 5-A-RU treated mice, we found statistically significant associations between BALF MAIT frequency and BALF OSP-IgG antibody responses (P=0.0389) and lung MAIT frequency and serum OSP-IgM antibody responses (P=0.0287) (Supplementary Table 1). Based on previous reports by our group and others showing IL-21-producing MAIT cells in humans [15, 17], we hypothesized that higher IL-21 production by expanded MAIT cells in the 5-A-RU group compared to the MGO only group may be responsible for the higher BALF OSP-IgG antibodies. To test this hypothesis, we measured IL-21 concentration in supernatant from BALF (Supplementary Figure 4F) and lung homogenate (Supplementary Figure 4G). We report no differences in IL-21 concentration between the 2 groups. In addition to measuring antibody responses, we also used flow cytometry to analyze B-cell differentiation and class switching in lung, BALF, and MLN. Specifically, we analyzed the frequencies of total B220+ B cells (Figure 5G-I), IgD+ naive B cells (Figure 5J-L), and IgD- CD38high class-switched memory B cells (Figure 5M-O) in MGO compared to 5-A-RU groups. We found no statistically significant changes in B-cell subtypes in all tissues. Together, these data show that low-dose intranasal 5-A-RU with MGO can expand MAIT cells in the mucosa of mice and moderately enhance mucosal IgG polysaccharide responses despite little effect on systemic antibody and B-cell differentiation.

Figure 4. MAIT cells expand following a low-dose 5-A-RU treatment combined with intranasal V. cholerae OSP. (A) Intranasal MAIT ligand plus V. cholerae O1 Ogawa OSP:BSA vaccination timeline. (B) Representative FACS plots of MAIT cells (CD44High MR1-Tetramer+) from lung, BALF, and MLN gated off Live B220- TCRβ+ cells. (C-E) Frequency of MAIT (left), CD8 (middle), and CD4 (right) T cells as a percentage of TCRb+ cells in (C) lung, (D) BALF, and (E) MLN. Data are represented as Median with IQR from 2 independent experiments. n=8-10 mice per group. *P < 0.05, **P > 0.01, ***P > 0.001, ***P > 0.0001 by 2-tailed Mann-Whitney U test.
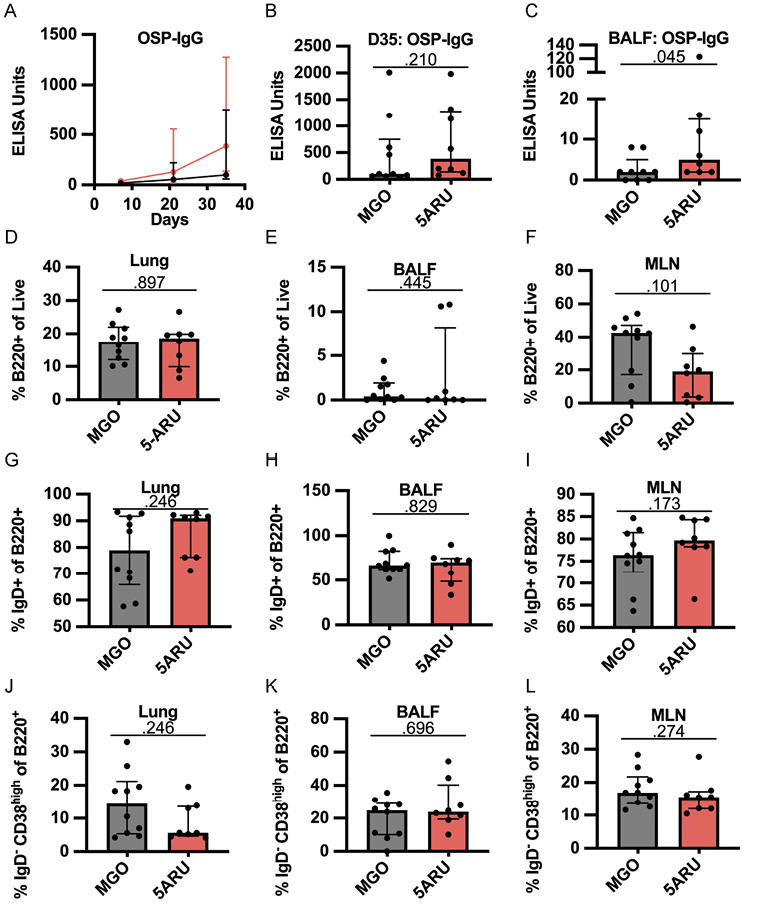
Figure 5. Intranasal 5-A-RU administration results in moderately higher mucosal polysaccharide responses. (A) Serum time-course, (B) serum day 35 endpoint, and (C) BALF day 35 endpoint OSP-IgG ELISAs. Data are represented as ELISA units measured kinetically and normalized to positive control pooled serum from WT B6 mice intranasally vaccinated with live V. cholerae. (D-F) Frequency of total B cells (B220+) as a percentage of live cells in (D) lung, (E) BALF, and (F) MLN. (G-I) Frequency of naive B cells (IgD+) as a percentage of B220+ cells in (G) lung, (H) BALF, and (I) MLN. (J-L) Frequency of class-switched memory B cells (IgD- CD38High) as a percentage of B220+ cells in (J) lung, (K) BALF, and (L) MLN. Data are represented as Median with IQR from 2 independent experiments. n=8-10 mice per group. P values determined by 2-tailed Mann-Whitney U test.
The recent discovery and accessibility of MR1-binding and MAIT-activating ligands has led to research into potential approaches targeting MAIT cells for both therapeutic and prophylactic treatments and vaccines against cancer [44, 52, 53], viruses [48], and bacteria [39–42, 45, 54]. Our study focuses on targeting MAIT cells in the context of cholera vaccines, which have limited long-term efficacy, particularly in young children [22, 55, 56]. In this study, we used murine models of cholera vaccination to test the adjuvant effects of targeting MAIT cells via intranasal administration of 5-A-RU with MGO. While we demonstrated significant expansion and persistence of mucosal MAIT cells through high- and low-dose intranasal 5-A-RU treatment, we saw limited 5-A-RU adjuvant effects on V. cholerae-specific antibody responses and B-cell differentiation.
We found higher mucosal OSP-IgG antibodies in low-dose 5-A-RU treated mice compared to MGO controls (Figure 5C), accompanied by a non-statistically significant higher serum OSP-IgG (Figure 5A and B). Additionally, we found significant correlations with BALF MAIT cell frequency and BALF OSP-IgG antibody responses (Supplementary Table 1). These results are important in the context of cholera, as OSP-IgG and IgA antibodies are associated with protection from subsequent cholera infection, and recently were shown to inhibit V. cholerae motility in vitro [50, 51, 57]. Furthermore, these data build on previous reports associating human MAIT cells with polysaccharide-specific B-cell responses, including after Shigella dysenteriae vaccination [11], and following V. cholerae O1 infection, where MAIT cells were correlated with LPS but not CT-specific antibody responses [10]. The mechanism of this MAIT B-cell help remains unknown despite studies by our group and others implicating MAIT cells in humoral responses in mice, non-human primates, and humans [12–15, 17].
MAIT cells may help B cells via contact dependent or contact independent interactions. In support of contact dependent MAIT B-cell interactions, human B cells activate MAIT cells in vitro via MR1 leading to increased frequency of inflammatory cytokine and CD40 Ligand (CD40L) producing MAIT cells [35, 58], and B cells are required for MAIT cell development in the mouse periphery [33]. Furthermore, murine MAIT cells promote B-cell autoantibody production in vitro in a mouse model of lupus via CD40L:CD40 signaling [13]. Based on these data, there is a potential for mouse MAIT cells to directly provide help to B cells via TCR:MR1 and CD40:CD40L co-stimulation. Alternatively, MAIT cells may provide B-cell help via indirect interactions. For example, human MAIT cells in tuberculosis pleural effusions [15] and tonsils [17] have been shown to produce IL-21, a key cytokine in B-cell differentiation, proliferation, and class switching [59], although this has not been confirmed in mice, and we did not find any differences in mucosal IL-21 concentration between treatment groups in our OSP vaccination model (Supplementary Figure 4F-G). MAIT cells have also been implicated in dendritic cell (DC) maturation via CD40L [60] and GM-CSF [61] production, suggesting DC licensing may play a role in B-cell help. This is supported by Pankhurst et al [48], which outlines a model of improved Tfh and germinal center responses to influenza HA antigen following CD40L-mediated MAIT licensing of DCs. As we used OSP conjugated to BSA (a protein antigen) it is possible enhancement of T-cell responses via MAIT cell CD40L-induced DC licensing was responsible for our phenotype.
Although optimization of the timing and dosing of 5-A-RU and OSP may yield more noteworthy outcomes, we hypothesize that an OSP:MAIT-ligand conjugate may be a more viable strategy to improve polysaccharide responses. This strategy has proven protective in a similar mouse model using the iNKT agonist, α-GC, conjugated to Streptococcus pneumoniae capsule polysaccharide [62]. In general, conjugate vaccines have been targeted to encapsulated bacteria including Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae using bacterial capsular polysaccharides conjugated to strong protein antigens such as tetanus toxin to induce germinal center B:T cell interactions and long-lived plasma and memory B cells [63]. Similarly, V. cholerae O1 OSP conjugate vaccines have also been tested in mouse models, where intramuscular or intradermal injections of OSP conjugated to tetanus toxin but not OSP alone induced anti-OSP IgG but not IgA antibodies and these injections were protective in a V. cholerae neonatal challenge model [49, 64]. As V. cholerae is a non-invasive pathogen, protection is largely driven by secreted IgM and IgA antibodies against OSP, potentially through inhibition of bacterial motility [51, 57], thus induction of mucosal IgM and IgA is critical. Alternatively, targeting MAIT cells using an OSP:MAIT-ligand conjugate vaccine may hold promise to improve mucosal IgA responses as MAIT cells are enriched in the mucosa and have been shown by our group to boost V. cholerae-specific IgA antibodies in a mouse MAIT adoptive transfer model [17].
Other than higher mucosal OSP-IgG titers in our OSP vaccination model, we did not see significant differences in any other measures of the humoral immune responses we examined. Specifically, analysis of B-cell and non-MAIT T-cell frequencies, and serum and mucosal antibodies, showed no significant differences between our control and 5-A-RU treated groups in either our live V. cholerae or OSP vaccination models. As our flow-based analysis was limited to cells harvested at the experiment’s endpoint, it is possible that differences at earlier time points may not be detected, though serum antibody data from both models indicate few differences. These data underscore the need for more in-depth studies regarding the mechanism of B-cell help, and the kinetics of such. Mucosal 5-OP-RU has been utilized in both mouse and rhesus macaque models as a vaccine adjuvant or therapeutic treatment with varying success. Expansion of MAIT cells using intranasal 5-OP-RU reduced lung bacterial load in models of Legionella longbeachae [40] and challenge with Mycobacterium bovis Bacillus Calmette-Guerin [54], and 5-OP-RU therapeutic vaccination of mice chronically infected with Mycobacterium tuberculosis reduced bacterial load in the lung, although prophylactic vaccination had a detrimental effect due to delayed CD4+ T-cell priming [41]. In contrast, intratracheal administration of 5-OP-RU in macaques infected with M. tuberculosis resulted only in exhaustion of MAIT cells, with no evidence of expansion, and no impact on clinical or microbiologic outcomes [45]. In the context of these prior studies, our data highlight the potential limitations of 5-OP-RU as a bacterial mucosal vaccine adjuvant, although its use in humans remain largely unexplored.
Our study has a number of limitations. First, given the paucity of differences in polysaccharide-specific immune responses between ligand-adjuvanted and non-adjuvanted groups, we did not exhaustively explore the kinetics or the dose-dependency of the limited phenotype we observed in this model. We also did not proceed with any infectious challenge studies to examine its impact on protection or bacterial burden. Second, our studies were performed in a murine model, and the composition of murine MAIT cells may not approximate those in humans. For example, while mice have a clear differentiation of Th1-like and Th17-like MAIT cells [8], such a dichotomy is not evident in human MAIT cells, which possess a wide transcriptional landscape [43]. In addition, relative to humans, mice have a much higher proportion of iNKT cells in the mucosa potentially limiting the MAIT adjuvant phenotype in this tissue niche [30–32]. We also did not examine the transcriptional programming of expanded MAIT cells in our models, although previous studies have shown an increase in IL-17A and IFN-g producing MAIT cells following intranasal 5-OP-RU plus Pam2 [42]. Third, we did not test the adjuvant effect of 5-OP-RU in a systemic vaccine model. Although in mice splenic MAIT cell frequency is low [8], targeting splenic MAIT cells via systemic vaccination, such as with a conjugate OSP-MAIT ligand vaccine, may be a viable strategy for more direct enhancement of MAIT-B cell reactions within germinal centers. Fourth, we report very low OSP-IgA antibody responses (Supplementary Figure 4C and D) in the mucosa and serum despite the mucosal nature of our OSP:BSA vaccine model. This is in contrast to our live V. cholerae challenge model (Figure 1A) which induced robust mucosal OSP-IgA responses (Figure 2H), and to studies showing markedly increased OSP antibodies following cholera infection compared to vaccination [51, 56]. Thus, this may be a suboptimal model to investigate polysaccharide-specific responses, although it highlights the difficulties of mucosal vaccine development particularly to non-protein antigens and need for mucosal adjuvants to enhance these responses.
As cholera and other mucosal bacterial infections remain a significant public health burden, the need for improved mucosal vaccine strategies is vital. We sought to investigate whether targeting MAIT cells is a viable option to improve antibody responses to V. cholerae vaccinations. Despite clear MAIT cell expansion following MAIT ligand treatment we found limited effects on adaptive immune responses. In conclusion, our study highlights both the potential and limitations of targeting MAIT cells with mucosal adjuvants to improve bacterial vaccines and adds to the growing body of work investigating MAIT ligands in both therapeutic and prophylactic vaccines.
We would like to thank the staff of the University of Utah Office of Comparative Medicine and Flow Cytometry Cores.
This work was supported by the NIH (R01 Al130378 to D.T.L., T32 Al138945 to O.J. and R37 AI106878 to E.T.R.).
The authors declare that they have no competing interests.
Supplementary materials are available at the Pathogens and Immunity website. Supplementary data may be provided by the authors to benefit the reader. Supplementary data are not copyedited and are the sole responsibility of the authors. Questions or comments related to supplementary materials should be addressed to the corresponding author.
Supplementary Figures and Table
1. Tilloy F, Treiner E, Park SH, Garcia C, Lemonnier F, de la Salle H, Bendelac A, Bonneville M, Lantz O. An invariant T cell receptor alpha chain defines a novel TAP-independent major histocompatibility complex class Ib-restricted alpha/beta T cell subpopulation in mammals. J Exp Med. 1999;189(12):1907-21. doi: 10.1084/jem.189.12.1907. PubMed PMID: 10377186; PMCID: PMC2192962.
2. Reantragoon R, Corbett AJ, Sakala IG, Gherardin NA, Furness JB, Chen Z, Eckle SB, Uldrich AP, Birkinshaw RW, Patel O, Kostenko L, Meehan B, Kedzierska K, Liu L, Fairlie DP, Hansen TH, Godfrey DI, Rossjohn J, McCluskey J, Kjer-Nielsen L. Antigen-loaded MR1 tetramers define T cell receptor heterogeneity in mucosal-associated invariant T cells. J Exp Med. 2013;210(11):2305-20. doi: 10.1084/jem.20130958. PubMed PMID: 24101382; PMCID: PMC3804952.
3. Lepore M, Kalinichenko A, Colone A, Paleja B, Singhal A, Tschumi A, Lee B, Poidinger M, Zolezzi F, Quagliata L, Sander P, Newell E, Bertoletti A, Terracciano L, De Libero G, Mori L. Parallel T-cell cloning and deep sequencing of human MAIT cells reveal stable oligoclonal TCRbeta repertoire. Nat Commun. 2014;5:3866. doi: 10.1038/ncomms4866. PubMed PMID: 24832684.
4. Treiner E, Duban L, Bahram S, Radosavljevic M, Wanner V, Tilloy F, Affaticati P, Gilfillan S, Lantz O. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature. 2003;422(6928):164-9. doi: 10.1038/nature01433. PubMed PMID: 12634786.
5. Kjer-Nielsen L, Patel O, Corbett AJ, Le Nours J, Meehan B, Liu L, Bhati M, Chen Z, Kostenko L, Reantragoon R, Williamson NA, Purcell AW, Dudek NL, McConville MJ, O’Hair RA, Khairallah GN, Godfrey DI, Fairlie DP, Rossjohn J, McCluskey J. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature. 2012;491(7426):717-23. doi: 10.1038/nature11605. PubMed PMID: 23051753.
6. Patel O, Kjer-Nielsen L, Le Nours J, Eckle SB, Birkinshaw R, Beddoe T, Corbett AJ, Liu L, Miles JJ, Meehan B, Reantragoon R, Sandoval-Romero ML, Sullivan LC, Brooks AG, Chen Z, Fairlie DP, McCluskey J, Rossjohn J. Recognition of vitamin B metabolites by mucosal-associated invariant T cells. Nat Commun. 2013;4:2142. doi: 10.1038/ncomms3142. PubMed PMID: 23846752.
7. Dusseaux M, Martin E, Serriari N, Peguillet I, Premel V, Louis D, Milder M, Le Bourhis L, Soudais C, Treiner E, Lantz O. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood. 2011;117(4):1250-9. doi: 10.1182/blood-2010-08-303339. PubMed PMID: 21084709.
8. Rahimpour A, Koay HF, Enders A, Clanchy R, Eckle SB, Meehan B, Chen Z, Whittle B, Liu L, Fairlie DP, Goodnow CC, McCluskey J, Rossjohn J, Uldrich AP, Pellicci DG, Godfrey DI. Identification of phenotypically and functionally heterogeneous mouse mucosal-associated invariant T cells using MR1 tetramers. J Exp Med. 2015;212(7):1095-108. doi: 10.1084/jem.20142110. PubMed PMID: 26101265; PMCID: PMC4493408.
9. Lamichhane R, Schneider M, de la Harpe SM, Harrop TWR, Hannaway RF, Dearden PK, Kirman JR, Tyndall JDA, Vernall AJ, Ussher JE. TCR- or Cytokine-Activated CD8(+) Mucosal-Associated Invariant T Cells Are Rapid Polyfunctional Effectors That Can Coordinate Immune Responses. Cell Rep. 2019;28(12):3061-76 e5. doi: 10.1016/j.celrep.2019.08.054. PubMed PMID: 31533031.
10. Leung DT, Bhuiyan TR, Nishat NS, Hoq MR, Aktar A, Rahman MA, Uddin T, Khan AI, Chowdhury F, Charles RC, Harris JB, Calderwood SB, Qadri F, Ryan ET. Circulating mucosal associated invariant T cells are activated in Vibrio cholerae O1 infection and associated with lipopolysaccharide antibody responses. PLoS Negl Trop Dis. 2014;8(8):e3076. doi: 10.1371/journal.pntd.0003076. PubMed PMID: 25144724; PMCID: PMC4140671.
11. Le Bourhis L, Dusseaux M, Bohineust A, Bessoles S, Martin E, Premel V, Core M, Sleurs D, Serriari NE, Treiner E, Hivroz C, Sansonetti P, Gougeon ML, Soudais C, Lantz O. MAIT cells detect and efficiently lyse bacterially-infected epithelial cells. PLoS Pathog. 2013;9(10):e1003681. doi: 10.1371/journal.ppat.1003681. PubMed PMID: 24130485; PMCID: PMC3795036.
12. Bennett MS, Trivedi S, Iyer AS, Hale JS, Leung DT. Human mucosal-associated invariant T (MAIT) cells possess capacity for B cell help. J Leukoc Biol. 2017;102(5):1261-9. doi: 10.1189/jlb.4A0317-116R. PubMed PMID: 28807929; PMCID: PMC5636046.
13. Murayama G, Chiba A, Suzuki H, Nomura A, Mizuno T, Kuga T, Nakamura S, Amano H, Hirose S, Yamaji K, Suzuki Y, Tamura N, Miyake S. A Critical Role for Mucosal-Associated Invariant T Cells as Regulators and Therapeutic Targets in Systemic Lupus Erythematosus. Front Immunol. 2019;10:2681. doi: 10.3389/fimmu.2019.02681. PubMed PMID: 31849932; PMCID: PMC6895065.
14. Rahman MA, Ko EJ, Bhuyan F, Enyindah-Asonye G, Hunegnaw R, Helmold Hait S, Hogge CJ, Venzon DJ, Hoang T, Robert-Guroff M. Mucosal-associated invariant T (MAIT) cells provide B-cell help in vaccinated and subsequently SIV-infected Rhesus Macaques. Sci Rep. 2020;10(1):10060. doi: 10.1038/s41598-020-66964-0. PubMed PMID: 32572140; PMCID: PMC7308357.
15. Jiang J, Cao Z, Qu J, Liu H, Han H, Cheng X. PD-1-expressing MAIT cells from patients with tuberculosis exhibit elevated production of CXCL13. Scand J Immunol. 2020;91(4):e12858. doi: 10.1111/sji.12858. PubMed PMID: 31833092.
16. Provine NM, Amini A, Garner LC, Spencer AJ, Dold C, Hutchings C, Silva Reyes L, FitzPatrick MEB, Chinnakannan S, Oguti B, Raymond M, Ulaszewska M, Troise F, Sharpe H, Morgan SB, Hinks TSC, Lambe T, Capone S, Folgori A, Barnes E, Rollier CS, Pollard AJ, Klenerman P. MAIT cell activation augments adenovirus vector vaccine immunogenicity. Science. 2021;371(6528):521-6. doi: 10.1126/science.aax8819. PubMed PMID: 33510029; PMCID: PMC7610941.
17. Jensen O, Trivedi S, Meier JD, Fairfax KC, Hale JS, Leung DT. A subset of follicular helper-like MAIT cells can provide B cell help and support antibody production in the mucosa. Sci Immunol. 2022;7(67):eabe8931. doi: 10.1126/sciimmunol.abe8931. PubMed PMID: 35030034; PMCID: PMC9001248.
18. Boulouis C, Kammann T, Cuapio A, Parrot T, Gao Y, Mouchtaridi E, Wullimann D, Lange J, Chen P, Akber M, Rivera Ballesteros O, Muvva JR, group Cs, Smith CIE, Vesterbacka J, Kieri O, Nowak P, Bergman P, Buggert M, Ljunggren HG, Aleman S, Sandberg JK. MAIT cell compartment characteristics are associated with the immune response magnitude to the BNT162b2 mRNA anti-SARS-CoV-2 vaccine. Mol Med. 2022;28(1):54. doi: 10.1186/s10020-022-00484-7. PubMed PMID: 35562666; PMCID: PMC9100314.
19. Ali M, Nelson AR, Lopez AL, Sack DA. Updated global burden of cholera in endemic countries. PLoS Negl Trop Dis. 2015;9(6):e0003832. doi: 10.1371/journal.pntd.0003832. PubMed PMID: 26043000; PMCID: PMC4455997.
20. World Health O. Cholera vaccine: WHO position paper, August 2017 - Recommendations. Vaccine. 2018;36(24):3418-20. doi: 10.1016/j.vaccine.2017.09.–34. PubMed PMID: 29555219.
21. Kauffman RC, Bhuiyan TR, Nakajima R, Mayo-Smith LM, Rashu R, Hoq MR, Chowdhury F, Khan AI, Rahman A, Bhaumik SK, Harris L, O’Neal JT, Trost JF, Alam NH, Jasinskas A, Dotsey E, Kelly M, Charles RC, Xu P, Kovac P, Calderwood SB, Ryan ET, Felgner PL, Qadri F, Wrammert J, Harris JB. Single-Cell Analysis of the Plasmablast Response to Vibrio cholerae Demonstrates Expansion of Cross-Reactive Memory B Cells. mBio. 2016;7(6). doi: 10.1128/mBio.02021-16. PubMed PMID: 27999163; PMCID: PMC5181778.
22. Bi Q, Ferreras E, Pezzoli L, Legros D, Ivers LC, Date K, Qadri F, Digilio L, Sack DA, Ali M, Lessler J, Luquero FJ, Azman AS, Cavailler P, Date K, Sreenivasan N, Matzger H, Luquero F, Grais R, Wiesner L, Ko M, Rouzier V, Peak C, Qadri F, Landegger J, Lynch J, Azman A, Sack D, Henkens M, Ciglenecki I, Ivers L, Diggle E, Weiss M, Hinman A, Maina K, Mirza I, Gimeno G, Levine M. Protection against cholera from killed whole-cell oral cholera vaccines: a systematic review and meta-analysis. The Lancet Infectious Diseases. 2017;17(10):1080-8. doi: 10.1016/s1473-3099(17)30359-6.
23. Harris JB, LaRocque RC, Chowdhury F, Khan AI, Logvinenko T, Faruque AS, Ryan ET, Qadri F, Calderwood SB. Susceptibility to Vibrio cholerae infection in a cohort of household contacts of patients with cholera in Bangladesh. PLoS Negl Trop Dis. 2008;2(4):e221. doi: 10.1371/journal.pntd.0000221. PubMed PMID: 18398491; PMCID: PMC2271133.
24. Patel SM, Rahman MA, Mohasin M, Riyadh MA, Leung DT, Alam MM, Chowdhury F, Khan AI, Weil AA, Aktar A, Nazim M, LaRocque RC, Ryan ET, Calderwood SB, Qadri F, Harris JB. Memory B cell responses to Vibrio cholerae O1 lipopolysaccharide are associated with protection against infection from household contacts of patients with cholera in Bangladesh. Clin Vaccine Immunol. 2012;19(6):842-8. doi: 10.1128/CVI.00037-12. PubMed PMID: 22518009; PMCID: PMC3370438.
25. Leung DT, Uddin T, Xu P, Aktar A, Johnson RA, Rahman MA, Alam MM, Bufano MK, Eckhoff G, Wu-Freeman Y, Yu Y, Sultana T, Khanam F, Saha A, Chowdhury F, Khan AI, Charles RC, Larocque RC, Harris JB, Calderwood SB, Kovac P, Qadri F, Ryan ET. Immune responses to the O-specific polysaccharide antigen in children who received a killed oral cholera vaccine compared to responses following natural cholera infection in Bangladesh. Clin Vaccine Immunol. 2013;20(6):780-8. doi: 10.1128/CVI.00035-13. PubMed PMID: 23515016; PMCID: PMC3675980.
26. Cadoz M. Potential and limitations of polysaccharide vaccines in infancy. Vaccine. 1998;16(14-15):1391-5. doi: 10.1016/s0264-410x(98)00097-8.
27. Lavelle EC, Ward RW. Mucosal vaccines - fortifying the frontiers. Nat Rev Immunol. 2022;22(4):236-50. doi: 10.1038/s41577-021-00583-2. PubMed PMID: 34312520; PMCID: PMC8312369.
28. Li YQ, Yan C, Luo R, Liu Z. iNKT cell agonists as vaccine adjuvants to combat infectious diseases. Carbohydr Res. 2022;513:108527. doi: 10.1016/j.carres.2022.108527. PubMed PMID: 35240551.
29. Davitt CJH, Longet S, Albutti A, Aversa V, Nordqvist S, Hackett B, McEntee CP, Rosa M, Coulter IS, Lebens M, Tobias J, Holmgren J, Lavelle EC. Alpha-galactosylceramide enhances mucosal immunity to oral whole-cell cholera vaccines. Mucosal Immunol. 2019;12(4):1055-64. doi: 10.1038/s41385-019-0159-z. PubMed PMID: 30953000; PMCID: PMC7746523.
30. Wingender G, Kronenberg M. Role of NKT cells in the digestive system. IV. The role of canonical natural killer T cells in mucosal immunity and inflammation. Am J Physiol Gastrointest Liver Physiol. 2008;294(1):G1-8. doi: 10.1152/ajpgi.00437.2007. PubMed PMID: 17947447.
31. Saez de Guinoa J, Jimeno R, Gaya M, Kipling D, Garzon MJ, Dunn-Walters D, Ubeda C, Barral P. CD1d-mediated lipid presentation by CD11c(+) cells regulates intestinal homeostasis. EMBO J. 2018;37(5). doi: 10.15252/embj.201797537. PubMed PMID: 29378774; PMCID: PMC5830915.
32. Zeissig S, Kaser A, Dougan SK, Nieuwenhuis EE, Blumberg RS. Role of NKT cells in the digestive system. III. Role of NKT cells in intestinal immunity. Am J Physiol Gastrointest Liver Physiol. 2007;293(6):G1101-5. doi: 10.1152/ajpgi.00342.2007. PubMed PMID: 17717040.
33. Martin E, Treiner E, Duban L, Guerri L, Laude H, Toly C, Premel V, Devys A, Moura IC, Tilloy F, Cherif S, Vera G, Latour S, Soudais C, Lantz O. Stepwise development of MAIT cells in mouse and human. PLoS Biol. 2009;7(3):e54. doi: 10.1371/journal.pbio.1000054. PubMed PMID: 19278296; PMCID: PMC2653554 co-owns the filled patent for the 3C10 antibody. EM has received salary through this funding.
34. Tang XZ, Jo J, Tan AT, Sandalova E, Chia A, Tan KC, Lee KH, Gehring AJ, De Libero G, Bertoletti A. IL-7 licenses activation of human liver intrasinusoidal mucosal-associated invariant T cells. J Immunol. 2013;190(7):3142-52. doi: 10.4049/jimmunol.1203218. PubMed PMID: 23447689.
35. Jeffery HC, van Wilgenburg B, Kurioka A, Parekh K, Stirling K, Roberts S, Dutton EE, Hunter S, Geh D, Braitch MK, Rajanayagam J, Iqbal T, Pinkney T, Brown R, Withers DR, Adams DH, Klenerman P, Oo YH. Biliary epithelium and liver B cells exposed to bacteria activate intrahepatic MAIT cells through MR1. J Hepatol. 2016;64(5):1118-27. doi: 10.1016/j.jhep.2015.12.017. PubMed PMID: 26743076; PMCID: PMC4822535.
36. Tominaga K, Yamagiwa S, Setsu T, Kimura N, Honda H, Kamimura H, Honda Y, Takamura M, Yokoyama J, Suzuki K, Wakai T, Terai S. Possible involvement of mucosal-associated invariant T cells in the progression of inflammatory bowel diseases. Biomed Res. 2017;38(2):111-21. doi: 10.2220/biomedres.38.111. PubMed PMID: 28442662.
37. Hama I, Tominaga K, Yamagiwa S, Setsu T, Kimura N, Kamimura H, Wakai T, Terai S. Different distribution of mucosal-associated invariant T cells within the human cecum and colon. Cent Eur J Immunol. 2019;44(1):75-83. doi: 10.5114/ceji.2019.84020. PubMed PMID: 31114440; PMCID: PMC6526592.
38. Corbett AJ, Eckle SB, Birkinshaw RW, Liu L, Patel O, Mahony J, Chen Z, Reantragoon R, Meehan B, Cao H, Williamson NA, Strugnell RA, Van Sinderen D, Mak JY, Fairlie DP, Kjer-Nielsen L, Rossjohn J, McCluskey J. T-cell activation by transitory neo-antigens derived from distinct microbial pathways. Nature. 2014;509(7500):361-5. doi: 10.1038/nature13160. PubMed PMID: 24695216.
39. Chen Z, Wang H, D’Souza C, Sun S, Kostenko L, Eckle SB, Meehan BS, Jackson DC, Strugnell RA, Cao H, Wang N, Fairlie DP, Liu L, Godfrey DI, Rossjohn J, McCluskey J, Corbett AJ. Mucosal-associated invariant T-cell activation and accumulation after in vivo infection depends on microbial riboflavin synthesis and co-stimulatory signals. Mucosal Immunol. 2017;10(1):58-68. doi: 10.1038/mi.2016.39. PubMed PMID: 27143301.
40. Wang H, D’Souza C, Lim XY, Kostenko L, Pediongco TJ, Eckle SBG, Meehan BS, Shi M, Wang N, Li S, Liu L, Mak JYW, Fairlie DP, Iwakura Y, Gunnersen JM, Stent AW, Godfrey DI, Rossjohn J, Westall GP, Kjer-Nielsen L, Strugnell RA, McCluskey J, Corbett AJ, Hinks TSC, Chen Z. MAIT cells protect against pulmonary Legionella longbeachae infection. Nat Commun. 2018;9(1):3350. doi: 10.1038/s41467-018-05202-8. PubMed PMID: 30135490; PMCID: PMC6105587.
41. Sakai S, Kauffman KD, Oh S, Nelson CE, Barry CE, 3rd, Barber DL. MAIT cell-directed therapy of Mycobacterium tuberculosis infection. Mucosal Immunol. 2021;14(1):199-208. doi: 10.1038/s41385-020-0332-4. PubMed PMID: 32811991; PMCID: PMC7790750.
42. Vorkas CK, Levy O, Skular M, Li K, Aube J, Glickman MS. Efficient 5-OP-RU-Induced Enrichment of Mucosa-Associated Invariant T Cells in the Murine Lung Does Not Enhance Control of Aerosol Mycobacterium tuberculosis Infection. Infect Immun. 2020;89(1). doi: 10.1128/IAI.00524-20. PubMed PMID: 33077620; PMCID: PMC7927919.
43. Vorkas CK, Krishna C, Li K, Aube J, Fitzgerald DW, Mazutis L, Leslie CS, Glickman MS. Single-Cell Transcriptional Profiling Reveals Signatures of Helper, Effector, and Regulatory MAIT Cells during Homeostasis and Activation. J Immunol. 2022;208(5):1042-56. doi: 10.4049/jimmunol.2100522. PubMed PMID: 35149530; PMCID: PMC9012082.
44. Yan J, Allen S, McDonald E, Das I, Mak JYW, Liu L, Fairlie DP, Meehan BS, Chen Z, Corbett AJ, Varelias A, Smyth MJ, Teng MWL. MAIT Cells Promote Tumor Initiation, Growth, and Metastases via Tumor MR1. Cancer Discov. 2020;10(1):124-41. doi: 10.1158/2159-8290.CD-19-0569. PubMed PMID: 31826876.
45. Sakai S, Lora NE, Kauffman KD, Dorosky DE, Oh S, Namasivayam S, Gomez F, Fleegle JD, Tuberculosis Imaging P, Arlehamn CSL, Sette A, Sher A, Freeman GJ, Via LE, Barry Iii CE, Barber DL. Functional inactivation of pulmonary MAIT cells following 5-OP-RU treatment of non-human primates. Mucosal Immunol. 2021;14(5):1055-66. doi: 10.1038/s41385-021-00425-3. PubMed PMID: 34158594; PMCID: PMC8217205.
46. Li K, Vorkas CK, Chaudhry A, Bell DL, Willis RA, Rudensky A, Altman JD, Glickman MS, Aube J. Synthesis, stabilization, and characterization of the MR1 ligand precursor 5-amino-6-D-ribitylaminouracil (5-A-RU). PLoS One. 2018;13(2):e0191837. doi: 10.1371/journal.pone.0191837. PubMed PMID: 29401462; PMCID: PMC5798775.
47. Nygren E, Holmgren J, Attridge SR. Murine antibody responses following systemic or mucosal immunization with viable or inactivated Vibrio cholerae. Vaccine. 2008;26(52):6784-90. doi: 10.1016/j.vaccine.2008.10.011. PubMed PMID: 18951939.
48. Pankhurst TE, Buick KH, Lange JL, Marshall AJ, Button KR, Palmer OR, Farrand KJ, Stewart IFN, Bird T, Mason NC, Compton BJ, Comoletti D, Salio M, Cerundolo V, Painter GF, Hermans IF, Connor LM. MAIT cells activate dendritic cells to promote T follicular helper cell differentiation and humoral immunity. bioRxiv. 2022:1-37. doi: 10.1101/2022.03.31.486638.
49. Alam MM, Bufano MK, Xu P, Kalsy A, Yu Y, Freeman YW, Sultana T, Rashu MR, Desai I, Eckhoff G, Leung DT, Charles RC, LaRocque RC, Harris JB, Clements JD, Calderwood SB, Qadri F, Vann WF, Kovac P, Ryan ET. Evaluation in mice of a conjugate vaccine for cholera made from Vibrio cholerae O1 (Ogawa) O-specific polysaccharide. PLoS Negl Trop Dis. 2014;8(2):e2683. doi: 10.1371/journal.pntd.0002683. PubMed PMID: 24516685; PMCID: PMC3916310.
50. Aktar A, Rahman MA, Afrin S, Akter A, Uddin T, Yasmin T, Sami MIN, Dash P, Jahan SR, Chowdhury F, Khan AI, LaRocque RC, Charles RC, Bhuiyan TR, Mandlik A, Kelly M, Kovac P, Xu P, Calderwood SB, Harris JB, Qadri F, Ryan ET. Plasma and memory B cell responses targeting O-specific polysaccharide (OSP) are associated with protection against Vibrio cholerae O1 infection among household contacts of cholera patients in Bangladesh. PLoS Negl Trop Dis. 2018;12(4):e0006399. doi: 10.1371/journal.pntd.0006399. PubMed PMID: 29684006; PMCID: PMC5912711.
51. Islam K, Hossain M, Kelly M, Mayo Smith LM, Charles RC, Bhuiyan TR, Kovac P, Xu P, LaRocque RC, Calderwood SB, Simon JK, Chen WH, Haney D, Lock M, Lyon CE, Kirkpatrick BD, Cohen M, Levine MM, Gurwith M, Harris JB, Qadri F, Ryan ET. Anti-O-specific polysaccharide (OSP) immune responses following vaccination with oral cholera vaccine CVD 103-HgR correlate with protection against cholera after infection with wild-type Vibrio cholerae O1 El Tor Inaba in North American volunteers. PLoS Negl Trop Dis. 2018;12(4):e0006376. doi: 10.1371/journal.pntd.0006376. PubMed PMID: 29624592; PMCID: PMC5906022.
52. Petley EV, Koay HF, Henderson MA, Sek K, Todd KL, Keam SP, Lai J, House IG, Li J, Zethoven M, Chen AXY, Oliver AJ, Michie J, Freeman AJ, Giuffrida L, Chan JD, Pizzolla A, Mak JYW, McCulloch TR, Souza-Fonseca-Guimaraes F, Kearney CJ, Millen R, Ramsay RG, Huntington ND, McCluskey J, Oliaro J, Fairlie DP, Neeson PJ, Godfrey DI, Beavis PA, Darcy PK. MAIT cells regulate NK cell-mediated tumor immunity. Nat Commun. 2021;12(1):4746. doi: 10.1038/s41467-021-25009-4. PubMed PMID: 34362900; PMCID: PMC8346465.
53. Crowther MD, Dolton G, Legut M, Caillaud ME, Lloyd A, Attaf M, Galloway SAE, Rius C, Farrell CP, Szomolay B, Ager A, Parker AL, Fuller A, Donia M, McCluskey J, Rossjohn J, Svane IM, Phillips JD, Sewell AK. Genome-wide CRISPR-Cas9 screening reveals ubiquitous T cell cancer targeting via the monomorphic MHC class I-related protein MR1. Nat Immunol. 2020;21(2):178-85. doi: 10.1038/s41590-019-0578-8. PubMed PMID: 31959982; PMCID: PMC6983325.
54. Yu H, Yang A, Derrick S, Mak JYW, Liu L, Fairlie DP, Cowley S. Artificially induced MAIT cells inhibit M. bovis BCG but not M. tuberculosis during in vivo pulmonary infection. Sci Rep. 2020;10(1):13579. doi: 10.1038/s41598-020-70615-9. PubMed PMID: 32788608; PMCID: PMC7423888.
55. Qadri F, Ali M, Lynch J, Chowdhury F, Khan AI, Wierzba TF, Excler J-L, Saha A, Islam MT, Begum YA, Bhuiyan TR, Khanam F, Chowdhury MI, Khan IA, Kabir A, Riaz BK, Akter A, Khan A, Asaduzzaman M, Kim DR, Siddik AU, Saha NC, Cravioto A, Singh AP, Clemens JD. Efficacy of a single-dose regimen of inactivated whole-cell oral cholera vaccine: results from 2 years of follow-up of a randomised trial. The Lancet Infectious Diseases. 2018;18(6):666-74. doi: 10.1016/s1473-3099(18)30108-7.
56. Leung DT, Rahman MA, Mohasin M, Patel SM, Aktar A, Khanam F, Uddin T, Riyadh MA, Saha A, Alam MM, Chowdhury F, Khan AI, Charles R, LaRocque R, Harris JB, Calderwood SB, Qadri F, Ryan ET. Memory B cell and other immune responses in children receiving two doses of an oral killed cholera vaccine compared to responses following natural cholera infection in Bangladesh. Clin Vaccine Immunol. 2012;19(5):690-8. doi: 10.1128/CVI.05615-11. PubMed PMID: 22441386; PMCID: PMC3346319.
57. Charles RC, Kelly M, Tam JM, Akter A, Hossain M, Islam K, Biswas R, Kamruzzaman M, Chowdhury F, Khan AI, Leung DT, Weil A, LaRocque RC, Bhuiyan TR, Rahman A, Mayo-Smith LM, Becker RL, Vyas JM, Faherty CS, Nickerson KP, Giffen S, Ritter AS, Waldor MK, Xu P, Kovac P, Calderwood SB, Kauffman RC, Wrammert J, Qadri F, Harris JB, Ryan ET. Humans Surviving Cholera Develop Antibodies against Vibrio cholerae O-Specific Polysaccharide That Inhibit Pathogen Motility. mBio. 2020;11(6). doi: 10.1128/mBio.02847-20. PubMed PMID: 33203761; PMCID: PMC7683404.
58. Salerno-Goncalves R, Rezwan T, Sztein MB. B cells modulate mucosal associated invariant T cell immune responses. Front Immunol. 2014;4:511. doi: 10.3389/fimmu.2013.00511. PubMed PMID: 24432025; PMCID: PMC3882667.
59. Konforte D, Simard N, Paige CJ. IL-21: an executor of B cell fate. J Immunol. 2009;182(4):1781-7. doi: 10.4049/jimmunol.0803009. PubMed PMID: 19201828.
60. Salio M, Gasser O, Gonzalez-Lopez C, Martens A, Veerapen N, Gileadi U, Verter JG, Napolitani G, Anderson R, Painter G, Besra GS, Hermans IF, Cerundolo V. Activation of Human Mucosal-Associated Invariant T Cells Induces CD40L-Dependent Maturation of Monocyte-Derived and Primary Dendritic Cells. J Immunol. 2017;199(8):2631-8. doi: 10.4049/jimmunol.1700615. PubMed PMID: 28877992; PMCID: PMC5632842.
61. Meierovics AI, Cowley SC. MAIT cells promote inflammatory monocyte differentiation into dendritic cells during pulmonary intracellular infection. J Exp Med. 2016;213(12):2793-809. doi: 10.1084/jem.20160637. PubMed PMID: 27799620; PMCID: PMC5110023.
62. Cavallari M, Stallforth P, Kalinichenko A, Rathwell DC, Gronewold TM, Adibekian A, Mori L, Landmann R, Seeberger PH, De Libero G. A semisynthetic carbohydrate-lipid vaccine that protects against S. pneumoniae in mice. Nat Chem Biol. 2014;10(11):950-6. doi: 10.1038/nchembio.1650. PubMed PMID: 25282505.
63. Pollard AJ, Perrett KP, Beverley PC. Maintaining protection against invasive bacteria with protein-polysaccharide conjugate vaccines. Nat Rev Immunol. 2009;9(3):213-20. doi: 10.1038/nri2494. PubMed PMID: 19214194.
64. Sayeed MA, Bufano MK, Xu P, Eckhoff G, Charles RC, Alam MM, Sultana T, Rashu MR, Berger A, Gonzalez-Escobedo G, Mandlik A, Bhuiyan TR, Leung DT, LaRocque RC, Harris JB, Calderwood SB, Qadri F, Vann WF, Kovac P, Ryan ET. A Cholera Conjugate Vaccine Containing O-specific Polysaccharide (OSP) of V. cholerae O1 Inaba and Recombinant Fragment of Tetanus Toxin Heavy Chain (OSP:rTTHc) Induces Serum, Memory and Lamina Proprial Responses against OSP and Is Protective in Mice. PLoS Negl Trop Dis. 2015;9(7):e0003881. doi: 10.1371/journal.pntd.0003881. PubMed PMID: 26154421; PMCID: PMC4495926.
Submitted June 16, 2022 | Accepted July 14, 2022 | Published August 24, 2022
Copyright © The Author(s). This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License.