Table 2. Correlation of Inflammation Markers and Ectopic Fat Measures with Markers of Cardiometabolic Risk
|
SBP |
HbA1C |
HOMA-IR |
Total Cholesterol |
Triglycerides |
HDL |
Statin use |
||||||||
|
rho |
p |
rho |
p |
rho |
p |
rho |
p |
rho |
p |
rho |
p |
rho |
p |
|
|
IL-6 |
0.185 |
0.046 |
0.314 |
< 0.001 |
0.273 |
0.003 |
0.003 |
0.972 |
0.189 |
0.042 |
-0.011 |
0.910 |
-0.098 |
0.296 |
|
hs-CRP |
0.177 |
0.057 |
0.144 |
0.124 |
0.162 |
0.083 |
0.112 |
0.231 |
0.023 |
0.808 |
-0.058 |
0.539 |
-0.087 |
0.351 |
|
PCF |
0.192 |
0.042 |
0.220 |
0.020 |
0.466 |
< 0.001 |
0.047 |
0.623 |
0.170 |
0.074 |
-0.221 |
0.019 |
0.081 |
0.398 |
|
TAT |
0.208 |
0.028 |
0.278 |
0.003 |
0.409 |
< 0.001 |
0.084 |
0.380 |
0.161 |
0.091 |
-0.196 |
0.039 |
0.066 |
0.493 |
|
LA roof HU |
-0.287 |
0.002 |
-0.174 |
0.067 |
-0.262 |
0.005 |
-0.043 |
0.656 |
-0.116 |
0.223 |
0.102 |
0.286 |
-0.159 |
0.093 |
|
Peri-RCA HU |
-0.064 |
0.500 |
-0.028 |
0.771 |
0.025 |
0.792 |
-0.077 |
0.422 |
-0.027 |
0.776 |
-0.029 |
0.763 |
0.201 |
0.034 |
|
Liver HU |
-0.004 |
0.964 |
-0.088 |
0.360 |
-0.258 |
0.006 |
0.180 |
0.059 |
-0.094 |
0.329 |
0.175 |
0.067 |
-0.042 |
0.660 |
|
Color coding legend |
Negative correlation at |
Negative correlation at |
Negative correlation at |
P > 0.05 |
Positive correlation at |
Positive correlation at |
Positive correlation at |
|||||||
Table 3 displays the association of BMI and ectopic fat measures with (A) natural-log-transformed IL-6 and (B) natural-log-transformed hs-CRP values in unadjusted and adjusted linear regression models. In fully adjusted models, BMI was associated with both IL-6 (P = 0.001) and hs-CRP (P < 0.001). Scatter plots showed that BMI correlated with IL-6 (overall r = 0.308, P < 0.001) and hs-CRP concentrations (overall r = 0.389, P < 0.001); however, the strength of this association was significantly greater in women than in men (Figure 1A; for interaction in the fully adjusted model P =0.047 for IL-6 and P = 0.06 for hs-CRP). In contrast, there was no significant interaction by HIV status (Figure 1B; P for interactions > 0.3).
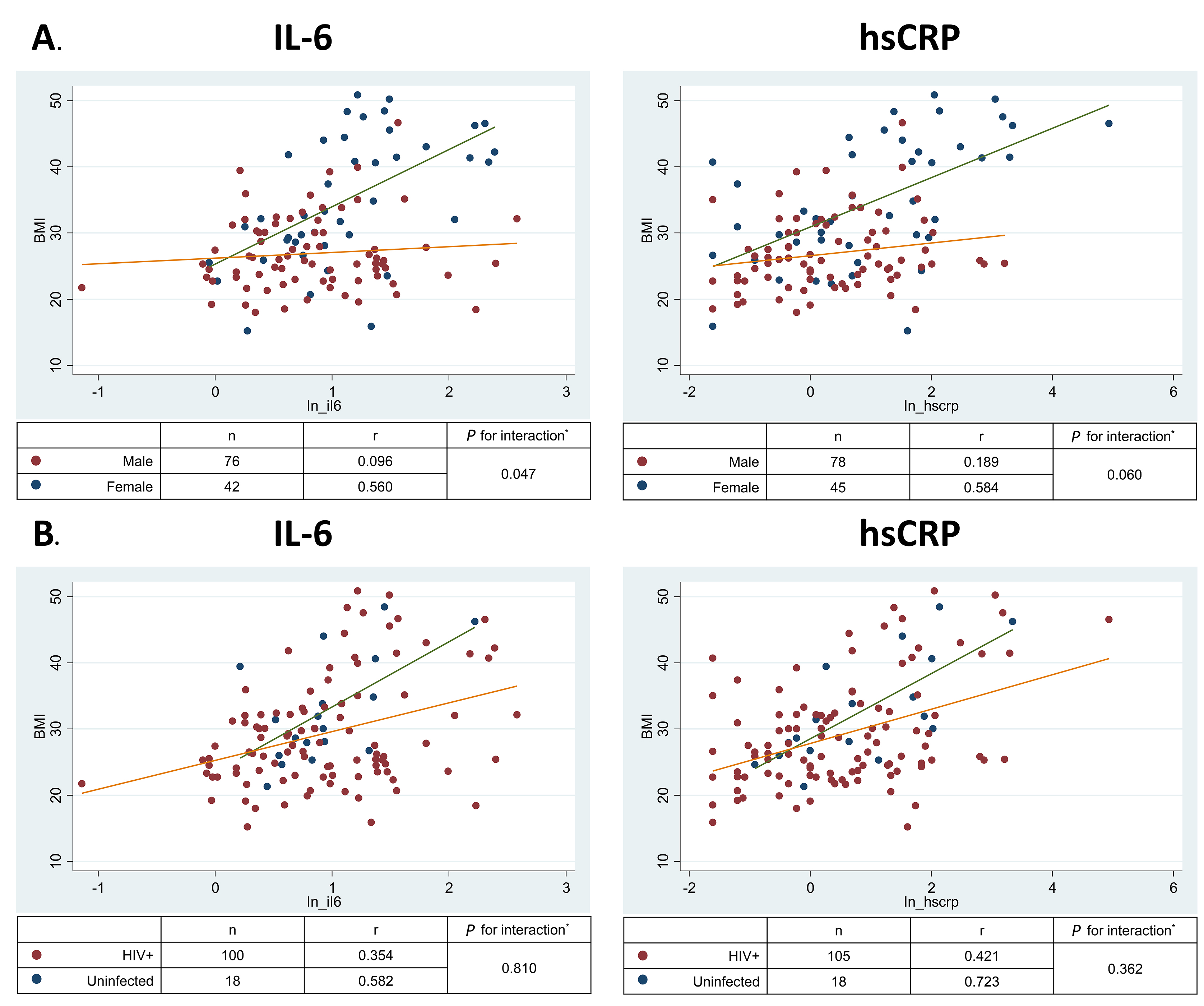
Figure 1. Relationship of BMI with biomarkers of inflammation by (A) sex and (B) HIV status. IL-6, Interleukin 6; hs-CRP, high sensitivity C reactive protein.
Table 3. Association of BMI and Ectopic Fat Measures with Natural-Log-Transformed IL-6 and hs-CRP in Unadjusted and Adjusted Linear Regression Models
|
|
|||||||||
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
BMI |
|
|
|
|
|
|
|
|
|
|
PCF |
|
|
|
|
|
|
|
|
|
|
TAT |
|
|
|
|
|
|
|
|
|
|
LA Roof |
|
|
|
|
|
|
|
|
|
|
Peri RCA |
|
|
|
|
|
|
|
|
|
|
Liver HU |
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
BMI |
|
|
|
|
|
|
|
|
|
|
PCF |
|
|
|
|
|
|
|
|
|
|
TAT |
|
|
|
|
|
|
|
|
|
|
LA Roof |
|
|
|
|
|
|
|
|
|
|
Peri RCA |
|
|
|
|
|
|
|
|
|
|
Liver HU |
|
|
|
|
|
|
|
|
|
PCF: pericardial fat, TAT: thoracic periaortic adipose tissue, LA Roof: left atrium roof, Peri-RCA: peri-right coronary artery # Fully adjusted model adjusts for age, sex, race, HIV status, insulin resistance (HOMA-IR), current smoking, and systolic blood pressure. *P < 0.05
Ectopic PCF and TAT volumes were positively associated with both IL-6 and hs-CRP (Table 3); however, adjustment for demographics and traditional risk factors somewhat attenuated these relationships. Furthermore, LA roof fat radiodensity (but not peri-RCA radiodensity) was inversely associated with hs-CRP in fully adjusted models and the association with IL-6 was borderline statistically significant (P = 0.054). In analyses of the sex-interaction, the magnitude of the inverse correlation between LA roof density and IL-6 and the positive correlation between PCF and IL-6 were stronger for women than for men (Figure 2A and 2B, interaction P=0.05 for both). Similar interactions were observed for hs-CRP, but were statistically weaker (P = 0.06 to 0.11). Sex interactions with TAT and peri-RCA radiodensity for both markers of inflammation were not significant (all P > 0.1). Mean liver attenuation was not consistently associated with either IL-6 or hs-CRP (Table 3; all P > 0.4).
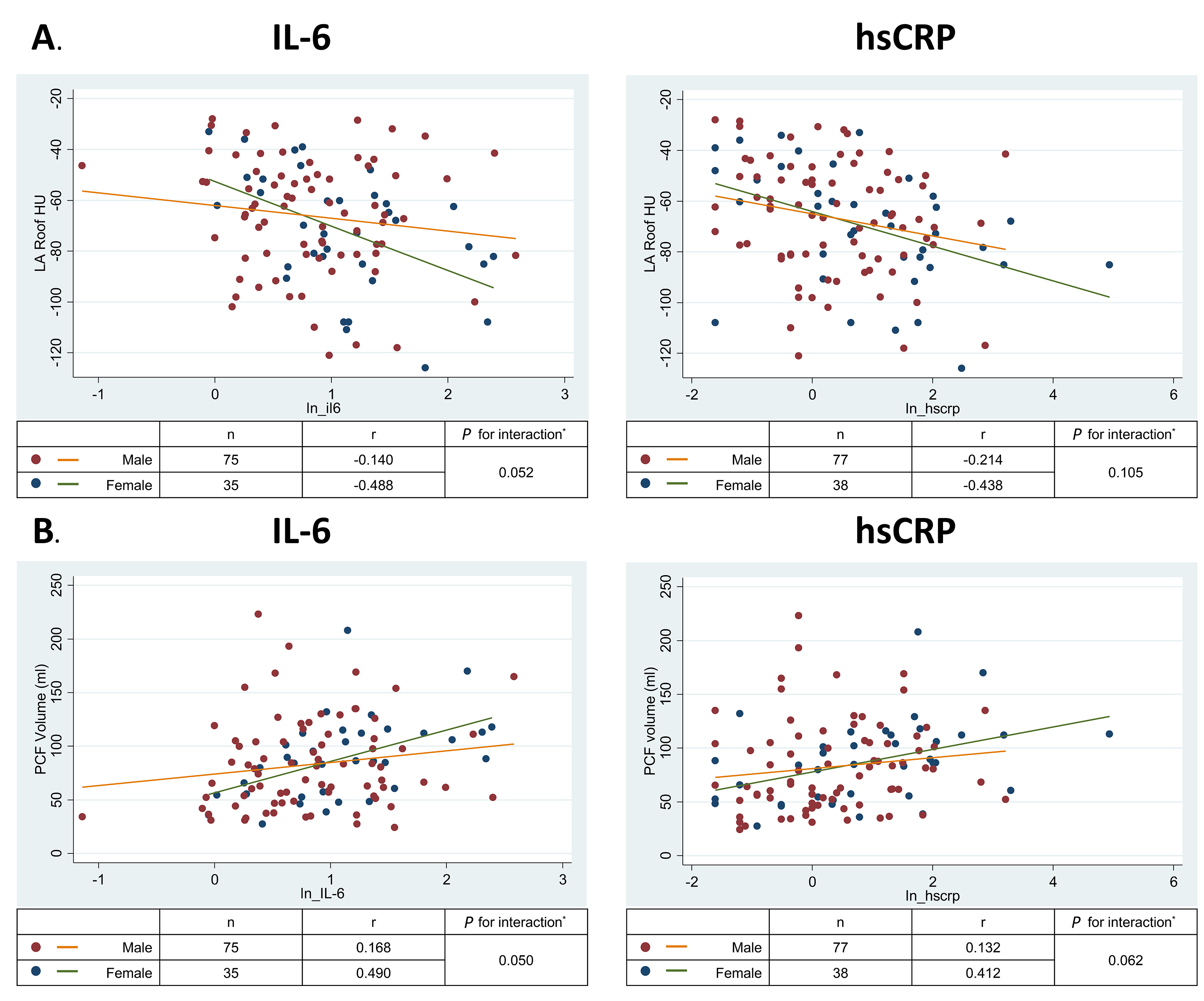
Figure 2. Relationship of left atrial roof pericardial fat density with interleukin-6 by sex
Discussion
Our findings demonstrate that among PWH receiving ART, total body adiposity and measures of ectopic fat volume and radiodensity are associated with circulating biomarkers of inflammation. We confirm and extend previous findings from our group [15, 16] by adding a measure of liver fat and regional assessment of PCF density in a new population of participants from a lifestyle intervention trial. Specifically, we show that total and ectopic fat burden appears to correlate with inflammation independently of traditional risk factors, and that the association of PCF density with inflammation may depend on the location of the measurement within the PCF fat depot. Importantly, we found evidence that the fat-inflammation relationship is stronger for women than men, but does not appear to differ by HIV status.
HIV infection and use of ART can lead to well-documented alterations in fat distribution and metabolic abnormalities [22]. The extent to which these pathological changes in fat structure and function are markers of or contribute in a causal way to chronic systemic inflammation and immune activation is an area of active research. If a phenotype of adiposopathy (ie “sick fat”) [23] can be identified among some with treated HIV infection using non-invasive imaging, then targeting these patients with lifestyle interventions and metabolic therapies may reduce the levels of chronic inflammation and immune activation that are thought to contribute to a wide range of non-AIDS chronic comorbidities such as cardiovascular disease and cancer.
An intervention that targets PCF specifically may have a greater impact on coronary heart disease, since it has been reported that PCF harbors proinflammatory cytokines that may directly influence the pathobiology of coronary atherosclerosis due to its close proximity and lack of fascial separation between the fat and arterial wall [24, 25]. We have previously shown that the PCF density is inversely associated with systemic inflammation [16]; however, in that study we measured global PCF density. The finding from this study that LA roof density is more strongly associated with systemic inflammation than peri-RCA fat may reflect regional differences in the association of PCF with the adiposopathy phenotype. This may represent differences in vascularity between a peri-coronary location and the LA roof [26]. Recent histologic studies [27] have confirmed that CT measured radiodensity of subcutaneous fat directly correlates with adiposity size among PWH receiving ART. Therefore, because pericardial fat is enriched with brown adipocytes that are smaller, more dense, and have high uncoupling protein-1 (UCP-1) expression [26], the pro-inflammatory adiposopathy phenotype may be reflected in the LA roof depot as a loss of UCP-1 activity, increased adipocyte size, and decreased PCF radiodensity. On the other hand, in the peri-RCA location, the correlation of radiodensity with adiposopathy may be confounded by local perivascular inflammation that increases radiodensity by increasing water content and fibrosis [20, 28].
Although sex and HIV status clearly affect fat distribution and function, whether the relationship between adiposity and inflammation differs by sex or HIV status is not clear [12, 29]. Many studies of PWH lack HIV-negative controls or sufficient numbers of female participants to evaluate these interactions. This appears to be the first study of PWH to show that the fat-inflammation relationship is stronger in women than in men. Our findings suggest that future observational studies and clinical trials of fat and inflammation in PWH should intentionally enroll enough women to allow for sex-stratified analyses. On the other hand, we did not find evidence that HIV status modifies the relationship between total body adiposity and inflammation. Although our study was nearly 40% female, the number of HIV-negative controls was small. Larger studies with more HIV-negative controls may be necessary to determine whether the fat-inflammation link is different in HIV-infected vs HIV-negative participants.
The other main limitation of this study is its cross-sectional nature, which may have resulted in residual confounding and does not allow for determination of causality. Additionally, we lacked detailed information on current individual ART drugs and drug classes. Because a recent clinical trial has suggested that differences in subcutaneous and visceral fat density change with different ART regimens [27], future studies should examine whether these differences are modified by sex. Finally, only a small proportion of participants met radiologic criteria for clinically significant hepatic steatosis, which may have limited our ability to detect relationships between liver fat and inflammation as has been reported by others [30].
Conclusions
Among PWH receiving ART, higher BMI and excessive visceral fat burden were associated with circulating markers of systemic inflammation. Because these measures appear to be more strongly related to inflammation among women than men, future clinical studies of metabolic risk and inflammation among PWH should include sex-stratified analyses.
Acknowledgments
MC analyzed the data and wrote the manuscript; CHY, CLH and ARW analyzed the data and revised the manuscript; CTL conceived of and supervised the study, analyzed data, and revised the manuscript. This study was funded by the American Heart Association (grant number 14CRP20380259 to ARW) and the National Institutes of Health (K23 HL123341 to CTL).